Global view on the pathogenesis of benign thyroid disease based on historical, experimental, biochemical and genetic data, identifying the role of magnesium, selenium, coenzyme Q10 and iron in the context of the unfolded protein response and protein quali
Abstract
We conducted a global review on the characteristics of benign thyroid disease beginning with the original clinical descriptions by Riedel, de Quervain and Hashimoto. A special section describes the attempts to fit human thyroid disease into animal models. The main emphasis is set on thyroglobulin including descriptions of isolation procedures and identification of molecular variations. The next element in this review describes the genetic knowledge about thyroglobulin synthesis and processing, showing that several mutations can result in a misfolded thyroglobulin molecule. This fact identifies the endoplasmic reticulum as a key element. Up to this point, thyroid disease still appears to be invincible. We proceeded to describe our own clinical experiences empirically aimed at improving mitochondrial function, i.e., ATP generation. We demonstrate several mechanisms, e.g., heat, stress and pregnancy, that can lead to an inflammatory thyroid condition. These changes can be reversed by supplementation with magnesium, selenium and coenzyme Q10. Finally, we reveal a functional relation between magnesium and anti-thyroglobulin antibody levels such that the antibodies disappear when magnesium levels normalize. We conclude that benign thyroid disease has the characteristics of an acquired mitochondrial dysfunction, which consequently compromises the function of the endoplasmic reticulum because of limited ATP supply. Altered thyroglobulin has the potential to change thyroid function on the basis of its autoregulatory thyroidal property. At the same time, it can be a stimulus for the production of anti-thyroglobulin antibodies. We propose a general applicability of this concept to beta-cell and ovarian function where compromised function of the endoplasmic reticulum can be expected.
Keywords
Introduction
Our commitment to medicine and medical research has always been inspired by the teachings and writings of many colleagues which we would like to present at the beginning. These research hints have paved our professional work through decades of clinical research. We have always looked back into historical accounts and have tried to bring them into the future. In a detailed narrative fashion, the reader will be confronted with historical data and valuable clinical descriptions as well as with advanced genetic investigations in the age of Precision Medicine.
Gregorio Marañón y Posadillo[1] - Spanish endocrinologist, born 1887, died 1960.
«Vivir no es solo existir, sino existir y crear, saber gozar y sufrir, y no dormir, sino soñar. Descansar es empezar a morir»
«Living is not just existing, but existing and creating, knowing how to enjoy and suffer, and not sleeping, but dreaming. To rest is to start to die»
Albert Szent-Györgyi (Bioenergetics, page 57 in[2]), Hungarian biochemist, born 1893, died 1986.
«Research is to see what everybody has seen and think what nobody has thought»
Christian Albert Theodor Billroth, German surgeon, born 1829, died 1894.
«Only the man who is familiar with the art and science of the past is competent to aid in its progress in the future»
A practical synthesis will consolidate the fundamental biochemical pieces into a general model of disease that is centered on the function of the endoplasmic reticulum and mitochondria. This concept will be demonstrated for thyroid diseases taking thyroglobulin as the main indicator. It will also be applied to diabetes and to reproductive physiology.
The main players: genetics in the time of precision medicine and the WOMED model of benign thyroid disease
With the introduction of precision medicine in 2015[3], research actions of the medical community have turned towards genetic methods to advance the understanding of mechanisms of disease, hoping to improve therapy options.
Long time before genetic details could be envisioned, medical research on thyroid disease had evolved according to the existing techniques since the 1890s. Early researchers investigating goiter concentrated on the clinical description of cases including their evolution and surgical therapy. Since the 1950s, experimental researchers have attempted to develop animal models that could resemble human disease, e.g., thyroiditis. In 1956, Danziger and Elmergreen presented a model of a homeostatic mechanism of the thyroid-pituitary relation based on a mathematical negative-feedback model[4], thus paving the way for the use of laboratory methods.
Over the past decades, developments of diagnostic methods have significantly improved and expanded the scope of clinical work. This has been achieved through accurate laboratory tests and imaging procedures. In spite of these advancements, subclinical thyroid disease was still being simply described as a biochemical condition in 2019[5]. Clinicians concerned about the recommendations launched by Bekkering[5] commented: “Thus, urgent search action is needed, not clinical inaction”[6], a comment that can be applied to the numerous clinical practice guidelines.
Our clinical work on benign thyroid diseases over the past 15 years has avoided inaction by introducing an advanced ultrasound methodology with 3D power Doppler imaging[7] and has gone through a step-by-step inclusion of additional laboratory parameters related to the new aspects found in sonography[8]. Power Doppler examination has allowed us to characterize an inflammatory condition common to subacute thyroiditis, hypothyroidism or hyperthyroidism related to a compound deficiency state affecting magnesium, coenzyme Q10 (CoQ10) and selenium levels. Our observational research results have allowed us to propose a model of acquired mitochondrial dysfunction as the central event in thyroid disease. Consequently, we designed a therapy concept aimed at clinical improvement of mitochondrial function[8,9]. This therapy can reverse the hypo-echogenic changes of the thyroid and also correct the inflammatory hyperperfusion of the gland[10]. Full therapy success needs at least 18 to 24 months of supplementation. Unfortunately, numerous clinical practice guidelines concentrate on hormone determinations, leaving no place for ultrasound or power Doppler.
The aim of this review was to look back in time and to analyze key findings in the field of benign thyroid diseases. By reading and extracting the original publications, we summarized historical, clinical, experimental, genetic and biochemical data. The synthesis of these findings will be used to further develop a new pathogenesis concept of thyroid disease based on the relation between magnesium levels and anti-thyroglobulin antibodies. This will bring us to a description of thyroglobulin synthesis including both specific and general aspects of the unfolded protein response as well as of the heat shock response.
Basic concepts of thyroid disease from Bernhard Riedel to Fritz de Quervain to Robert McCarrison and to Hakaru Hashimoto
In 1896, Riedel[11] presented his report on a special type of thyroid affection which was later called after him, Riedel thyroiditis. The publication started with the mention of 2 previous cases of chronic pancreatitis which presented as a large tumor surrounding the bile duct. Histological examination revealed chronic inflammation. With this background information, he then described 2 special cases of goiter which had been identified after having conducted thyroid surgery in 300 cases over 12 years. The first patient was a 42-year-old man who had noticed an enlargement of the thyroid over the past 6 months. The enlargement of the thyroid resulted in symptoms of local compression. The goiter firmly embraced both carotid arteries and the internal jugular veins. Histological examination revealed signs of inflammation with round cells. The second patient was a 23-year-old woman who presented with a fast-growing goiter over the past 10 weeks. This goiter showed a similar infiltration of the neck vessels as the previous case. Goiter growth stopped after surgery even though only one fifth of the gland was resected. In the discussion there is a mention of a similar case which had been observed in a 12-year-old girl.
In 1902, de Quervain[12] described a process of thyroid inflammation, which was later named after him, de Quervain thyroiditis. He differentiated the etiology of thyroiditis as being either purulent or non-purulent. Case 1 corresponded to a 16-year-old girl who had noticed a painful goiter which developed some 8 days after having had an angina. An emergency operation was necessary because of local compression problems. Histology showed no inflammatory changes, and the final diagnosis was that of a simple goiter. Neither the connective tissue nor vessels showed any changes. Case 2 described a 38-year-old woman who had also had an angina and who presented with a painful goiter. Case 3 described a 38-year-old woman who presented with a painful goiter which developed some 4½ months post-partum. Case 4 corresponded to a 40-year-old woman who presented with sudden swelling of the thyroid. A hemithyroidectomy of the left lobe was carried out. Histology revealed polynuclear leukocytes. The final diagnosis was parenchymal thyroiditis. A short phrase - lost amid these descriptions - stated that the pain symptoms of cases 2, 3 and 4 were successfully treated with salicylic acid, i.e., aspirin.
In 1908, McCarrison[13] described the histological appearance of the thyroid in a case of endemic cretinism demonstrating large fibrous strains together with a diminution of functional thyroid tissue (figure 5 in[13]).
In 1912, the classical description of lymphadenoid goiter was published by Hashimoto[14]. His report was based on the histological findings from 4 women who were thyroidectomized because of hypertrophic goiter. The histological analysis revealed no signs of acute inflammation. Speculating about the nature of the disease, Hashimoto proposed a process of chronic inflammation.
In 1931, Graham and McCullagh[15] described the association of lymphoid tissue with atrophy and fibrosis of the thyroid. The authors referred to the lack of publications related to the topic described by Hashimoto since 1912. They added the description of 4 cases of women with goiter who were seen at the Department of Surgical Pathology and Surgery of the Cleveland Clinic. The individual cases can be summarized as follows. Case 1, a 75-year-old woman with thyroid enlargement who was treated with radiotherapy 2 times. On surgery, both lobes were found to be enlarged. The gland was hard and fixed around the trachea, and no acute inflammation was seen. Case 2, a 44-year-old woman with diffuse enlargement of the gland. The gland was fibrotic and had no normal thyroid tissue. Case 3, a 53-year-old woman with long-standing goiter, which increased in size over the last 4 years. The thyroid expanded behind the trachea. The gland was fibrotic and contained no normal thyroid tissue. Case 4, a 75-year-old woman with a large goiter, which had been taken as malignant. The gland was fibrotic and had no normal thyroid tissue. In summary, the main characteristics were atrophy of the parenchyma, replacement fibrosis and lymphoid infiltration. Besides the presentation of these cases, the authors included an informative table that summarized the data taken from Hashimoto together with the data of Graham’s patients (page 561 in[15]). A pathogenic explanation, however, was not found. In the same year Graham published a comparison between Riedel’s struma and struma lymphomatosa[16]. The author collected the literature available at that time finding data from 104 cases (table 1 on page 64 in[16]). In the conclusions the author proposed that with time the changes of the thyroid become degenerative and sclerosing and that the lymphoid tissue “is non-specific for Hashimoto’s struma”.
The relationship between Riedel’s struma and struma lymphomatosa (Hashimoto) was described by Eisen in 1934[17]. He also collected the relevant literature of that time (references 4 to 12) making special reference to Ewing (1922) who considered Hashimoto’s thyroiditis to be an early stage of Riedel’s thyroiditis. Eisen’s conclusion was similar to that of Ewing when he stated: “Riedel’s struma and Hashimoto’s struma are different morphological manifestations of the same disease process”.
In 1935, a clinically oriented publication on Hashimoto’s disease by Clute, Eckerson and Warren appeared in a surgical journal[18]. They described the findings in 9 women with a mean age of 55.5 years. The main complaint was pressure on the midline structures, i.e., trachea. A common feature was extensive fibrosis, which was limited by the capsule, while inflammation was not described.
In 1937, McClintock and Wright[19] carried out a comparative study dealing with the overlap in the diagnosis of Riedel’s struma and Hashimoto’s disease. They reviewed the literature available at that time and were able to identify 60 patients with Riedel’s struma and 50 with Hashimoto’s disease (table 1 on page 12 in[19]). An additional group was that of patients who presented with atrophy and fibrosis. Tables 2 and 3 in[19] summarized the relations of each entity to sex, age, duration of symptoms, type of thyroid involvement and thyroid function. In Hashimoto’s thyroiditis, 95% of the cases were women. The duration of symptoms was much longer in Hashimoto’s thyroiditis. Thyroid involvement was unilateral in 30% of cases with Riedel’s thyroiditis, while it was bilateral in Hashimoto’s thyroiditis. The cases with atrophy had inflammatory fibrosis. Recovery after surgery for Riedel’s thyroiditis was rather rapid, while patients with Hashimoto’s thyroiditis needed a longer time to return to normal.
In 1941, Goodman[20] added a report on 2 cases of Riedel’s thyroiditis. The author emphasized that Hashimoto’s thyroiditis and Riedel’s thyroiditis must be distinguished and not taken as being similar. The wedge resection in cases of Riedel’s struma was recommended.
In 1942, Lundbæk reported the case of a woman, 41 years old, presenting with a moderate enlargement of the thyroid. The gland felt firm and hard but was not adherent. The histological diagnosis was Riedel’s struma. Treatment was given in the form of X-ray radiation administered 6 times at a dose of 100 r. The effect of this treatment was a diminution of goiter size[21]. This publication made mention of earlier studies on chronic thyroiditis[22], but unfortunately, we could find no details.
The clinical aspects of 24 cases presenting with chronic thyroiditis were described by Patterson and Starkey in 1948[23]. The authors stressed that a correct preoperative diagnosis was not always possible. After surgery, the final diagnosis revealed 11 cases of the Hashimoto type, 11 of the Riedel type, and 2 of the giant-cell type of de Quervain.
In 1951, Statland, Wasserman and Vickery, working at the Thyroid Clinic and Pathology Department of Massachusetts General Hospital, reported on 51 cases with Hashimoto’s thyroiditis described as struma lymphomatosa[24]. In the introduction, the authors made a clear differentiation between Riedel’s thyroiditis and Hashimoto’s struma, which led them to exclude cases with Riedel’s thyroiditis from their report. The frequency of struma lymphomatosa was 51 out of 3,676 thyroid operations. Forty-four of the cases were described as being typical for the disease, while 11 were not. The mean weight of the thyroid was 66.8 g compared to 20 g as seen in normal subjects in the fifth decade of life. The main complaint was enlargement in the neck. Thyroid tissue was described as being yellowish pink to tan and separated into lobulations separated by fibrous septa. The most outstanding feature was the diffuse distribution of lymphoid tissue and associated parenchymal degeneration. In their conclusions, they recognized that the cause for the diminished functional capacity of the altered thyroid cells was unknown.
The proposed “autoimmune” pathogenesis of human thyroid disease made a start with an extremely limited number of histological observations from surgical material (n = 3) in 1957[25]. Applying the medical knowledge available then and reviewed above, the true number of cases with chronic thyroiditis should have been reduced to one since one patient had thyroid carcinoma and the other patient had Riedel’s thyroiditis. Therefore, it turned out that a disease concept was launched as “a disease in one”. In spite of this severe medical and scientific limitation, this loose idea has been followed and investigated further by other researchers[26-28]. Despite research efforts, this concept has not produced any therapy approach in humans.
In 1959, Graham and Gilliland presented a case of a woman presenting with Riedel’s thyroiditis, where a hemithyroidectomy of the left lobe was required. In a follow-up examination, the right lobe appeared functional, showing 131iodine uptake[29].
Katz and Vickery[30] looked at Hashimoto’s thyroiditis from a pathologist’s point of view in 1974. They reviewed the material from the Massachusetts General Hospital available from 1931 until 1969. The sources of the material were: surgery (n = 16), needle biopsy (n = 40), open biopsy (n = 7), and autopsy (n = 2). The authors found fibrosis of the gland seen as broad bands of connective tissue which separated islands of thyroid parenchyma. Thirty percent of thyroid parenchyma was replaced by fibrous tissue (figures 2 to 4 in[30]). Working with the tanned red cell method for antibody detection they found anti thyroglobulin antibodies in many patients. These antibodies showed a correlation to situations of recent development of goiter as well as to fibrosis.
Experimental models of thyroid disease: lymphadenoid goiter, “autoimmunization” and the obese/obese strain of chickens
McCarrison[31] described in 1929 a model of experimental lymphadenoid goiter in rats. The animals received a dietary mixture consisting of white flour, meat residue, olive oil, and a salt mixture containing 0.45% potassium iodide. The resulting alterations in the thyroid were described as showing fibrosis and atrophy, i.e., regressive changes. The author concluded that dietary deficiencies, e.g., vitamins, could be considered to have caused the goiter.
The publication from Witebsky et al.[25] which proposed an “autoimmune” pathogenesis of human thyroid disease also presented experimental data to describe a so-called “autoimmunization” condition. Careful reading of the text reveals that this experiment was the product of a manipulated intervention, i.e., immunization, using organ extracts from rabbit thyroids together with Freund’s complete adjuvant. This - non physiological mixture - was applied to the footpads of the experimental animals. Freund’s adjuvant[32] is a toxic substance containing a mineral oil, Arlacel (mixture of mannide mono-oleate), together with killed Mycobacterium butyricum or Mycobacterium smegmatis. Our 2020 look at these data is critical since the words chosen for the title of the original publication suggested a natural connection between chronic thyroiditis in humans and autoimmunization in experimental animals. In the same publication, the authors referred to this situation as being used “under certain experimental conditions” (Page 1445 in[25]).
One important molecular issue identified by Schulman, Rose and Witebsky in 1955 was the alteration of thyroglobulin structure found after biochemical extraction[33]. The authors attributed this change to a shift in equilibrium between different aggregation states. This biochemical feature of thyroglobulin has not been followed in the literature.
In 1964, Weigle[34] described an experimental approach aimed at the induction of an immune reaction in rabbits. This publication also referred to “autoimmunity” even though it was clearly stated that the study was based on the use of - externally - altered tissue components, i.e., thyroglobulin. The alterations were produced by heating and coupling to diazonium derivatives. By using heat, 65 °C for 15 min, dissociated thyroglobulin was produced. Two slower sedimenting components, i.e., of lower molecular weight, were found. The diazonium derivatives were produced using either sulfanilic acid or arsanilic acid. This chemical intervention produced a breakdown of thyroglobulin into 4 components including 3 with a slower sedimentation. PubChem describes sulfanilic acid (4-aminobenzenesulfonic acid) as an irritant and arsanilic acid (4-aminophenyl arsonic acid) as being toxic and an environmental hazard. PubMed has no additional entries relating these substances to any “autoimmunization”.
In 1962, Van Tienhoven and Cole[35] described a complex disease including thyroiditis, which was observed in obese chickens. Many researchers have used this strain of chickens in further experiments. In 1994, Rose[36] summarized the topic of autoimmunity in relation to lessons from the birds. Careful reading of the publication discloses a conceptual pathogenesis paradox in the first lines when it is stated that autoimmune disease can be achieved by immunization. It could also be called a dedicated semantic mistake, i.e., dedicated to promoting the idea of autoimmunity. The self or auto intervention is physically and philosophically impossible since the experimental animals were actually vaccinated against foreign material. Rose coincides with our opinion when he declares and admits that “Autoimmune diseases can be produced in chickens, as they are in mammals, by experimental immunization” (page 984 in[36]). For these semantic reasons, we consider that the term autoimmune, referring to experimental immunization, is rather misleading just as fake news is today in 2020. Immunization is a process that involves external elements not found in the self[37].
Even though numerous publications have explored thyroid disease on the basis of the concept of autoimmunity as proposed by Rose, there has been no practical evolution into a therapy that would control the disease. Caturegli, a co-worker of Rose, provided a modern view on Hashimoto’s thyroiditis and included the following statement on therapy: “The treatment remains symptomatic and based on the administration of synthetic thyroid hormones to correct the hypothyroidism as needed”[38].
The following Table 1 summarizes some of the milestones of thyroid research including some hints as to how the views can change. In the following sections, we will look at the therapy attempts based on concepts of oxidative damage to the thyroid in relation to selenium deficiency. We will demonstrate some causes leading to thyroid inflammation and how this can be approached by prescribing a combined supplementation with magnesium, selenium and CoQ10[9,10].
Selected publications in the development of the pathogenesis concept of thyroiditis and goiter together with modern concepts
| Authors | Year | Topic |
|---|---|---|
| Riedel[11] | 1896 | Riedel’s thyroiditis |
| de Quervain[12] | 1902 | Description of acute thyroiditis and treatment with salicylic acid |
| McCarrison[13] | 1908 | Fibrotic thyroid in endemic cretinism, loss of functional thyroid tissue |
| Hashimoto[14] | 1912 | Original description of struma lymphomatosa |
| Rose[39] and Witebsky[40] | 1955 | Serological reactions with thyroid extracts, calling thyroglobulin a major player |
| Roitt[26,27,41] | 1958 | The idea of auto-antibodies |
| Van Tienhoven and Cole[35] | 1962 | Description of endocrine disturbances in obese chickens |
| Ritossa[42] | 1962 | Description of the heat shock response |
| Wartenberg et al.[43] | 1973 | Mitochondria in leukocyte migration inhibition test |
| Katz and Vickery[30] | 1974 | The fibrotic variant of Hashimoto thyroiditis |
| White and Walmsley[44] | 1984 | Plasma enzyme activities in 3 cases of hypothyroidism |
| Rose[36] | 1994 | Avian models of autoimmune disease |
| Medeiros-Neto et al.[45] | 1996 | Congenital hypothyroidism as an endoplasmic reticulum storage disease |
| Leo et al.[37] | 2011 | Fundamentals of immunization |
| Caturegli et al.[38] | 2014 | Therapeutic options for HT are either substitution or surgery |
| Jancic and Stosic[46] | 2014 | Cadmium toxicity in the thyroid via mitochondrial function |
| Zimmermann et al.[47] | 2016 | Alteration of the mitochondrial respiratory complex I in HT |
| Grootjans et al.[48] | 2016 | The unfolded protein response and immunization |
| Barić et al.[49] | 2019 | Relation between symptom burden and thyroglobulin antibodies |
| Wang et al.[50] | 2018 | Relation between magnesium levels and thyroglobulin antibodies |
| Hu et al.[51] | 2020 | Generalized concept of mitochondrial systems biology |
Oxidative damage to the thyroid and the potential use of selenium
On the basis of simple models of selenium involvement in protective mechanisms in the body as an anti-oxidant[52], some researchers have investigated the potential benefit of giving selenium as a single substance to treat thyroid disease, specially Hashimoto’s thyroiditis. A publication of the Cochrane Library explored the utility of selenium administration on Hashimoto’s disease in 2013[53], analyzing the data originating from 4 studies published between 2006 and 2011[54-57].
One aspect that was missed in the proposal of the evaluation, was that they did not select studies that had measured selenium. Keeping selenium levels as an unknown variable invalidates any conclusion. Invalidated analyses would not make Archie Cochrane happy since they cannot fulfill his ambitious postulate of finding a successful therapy in the sense of effectiveness and efficiency[58].
The citation in the Box 1 illustrates the inconclusive conclusions of the Cochrane analysis[53].
In the following paragraphs, we will refer to recent publications that have evaluated selenium supplementation in relation to thyroid disease. Between 2015 and 2017, 3 studies coming from Danish researchers appeared in the literature. In 2015, they reported the results of a double-blinded selenium supplementation trial[59]. The outcome measure was defined as changes of thyroid function, i.e., fT3, fT4 and TSH, as well as of thyroid peroxidase antibodies. The study used a patented selenium product SelenoPrecise (https://www.pharmanord.eu/) which is described by the producer as containing selenomethionine and more than 20 other organic selenium compounds. Selenium levels were determined at the beginning and during the supplementation period, 6 months and 5 years later. The doses administered were, 0, 100, 200 and 300 µg daily. Thyroid function parameters showed no change. A peculiar aspect of the study was the high total weight of the placebo preparation which adds up to 400 mg! (Page 660 in[59]). In 2016, a publication with a provocative title appeared. Referring to selenium, they posed the following question: “An element in search of the relevant indications?”[60]. The authors presented an analysis of a web-based survey done by the Italian Associazione Medici Endocrinologi in 2016[61]. The authors concluded that selenium use is inappropriate in several thyroid-related conditions. In 2017, Winther et al.[62] published a meta-analysis that looked at the efficacy of selenium supplementation in chronic autoimmune thyroiditis. The authors started by declaring that this disease has no cure but fail to add any validated reference for this statement. The notion of low selenium being associated with thyroid disease was taken from a previous Chinese publication[63]. Wu et al.[63] previously looked at thyroid function parameters, thyroid peroxidase antibodies, selenium, and thyroid volume determined by ultrasound examination using a 7.5-MHz probe. The authors found an association between an enlarged thyroid and low selenium levels. Unfortunately, this key clinical element was not considered in the analysis by Winther et al.[62]. In their opinion, selenium supplementation in patients with thyroid disease should be discouraged. They proposed carrying out well-powered randomised clinical trials (RCTs) evaluating disease progression or health-related quality of life. The evaluation of disease progression was not defined.
Mao et al.[64] studied the effect of low-dose selenium supplementation in pregnant women. They looked at parameters of thyroid function as well as at the levels of thyroid peroxidase antibodies. The study did not contain any data on selenium levels or on thyroid ultrasonography. The study failed to show an effect on antibody levels.
In 2017, Esposito et al.[65] studied the effects of short-term selenium supplementation including euthyroid subjects with Hashimoto’s thyroiditis. Thyroid parameters included were TSH, fT3, fT4, thyroid peroxidase antibodies, and thyroid echogenicity evaluated with a 10-MHz ultrasound probe. CXCL10 or interferon-gamma inducible protein 10 kD[66], originally considered to be involved in inflammatory processes[67], was included as a secondary outcome parameter. The authors concluded that short-time selenium supplementation was ineffective as no end-point parameter changed.
In 2020, a study on selenium supplementation in patients with subclinical hypothyroidism was published by Pirola et al.[68]. The study included the analysis of thyroid hormones and thyroid peroxidase antibodies, selenium and iodine. This study also included determinations of CXCL9, CXCL10 and CXCL11, taken as being representative for interferon-γ- inducible chemokines. The only change reported by the authors was normalization of TSH levels.
These recent publications coincide with the outcome of the Cochrane evaluation[53], i.e., the sole supplementation of selenium is not effective in modifying or treating thyroid disease.
Prior to the Cochrane analysis and in strong contrast to the results described above, Rani and Lalitha[69] in 1996 paved the road for selenium research in connection with mitochondrial function. They showed that electron transport function was impaired at the same time as mitochondrial structure was affected when selenium deficiency was present. We personally consider a mitochondrial involvement to be more important than any antioxidative presumption.
An interlude: updating the contribution of genetics
In 2011, Panicker[70] published an article on the genetics of thyroid disease. Some key statements are quoted here. “Although the advancement of genetic technology has led to many significant findings in the last decade or two, it is clear that we are only just beginning to understand the role of genetics in thyroid function and disease”. A second statement reads: “Early optimistic expectations that all genes responsible would be rapidly discovered have had to be reined in, and researchers across many fields are searching for the reasons this has not happened. The need for larger sample sizes and collaboration between groups with access to large cohorts has now been understood, and these studies will undoubtedly discover further genes. Furthermore, whole genome sequencing may provide more information as other types of genetic variation, such as copy number variants may also be found to play a role. In addition, influences on accessibility of genes to transcription may be at work. However, what we have already discovered has increased our understanding of normal thyroid hormone action and physiology, and we are beginning to understand the complex origins of autoimmune thyroid disease. Important for future work is the need to replicate the early findings presented above and perform functional studies to identify the true associations and the mechanisms behind them. These mechanisms will increase our understanding of thyroid physiology and identify therapeutic targets”. Also in 2011, Davies declared that there are no really significant genes for autoimmune thyroid disease[71].
Looking specifically at causes of Grave’s disease, Marinò et al.[72] concluded that the ultimate cause of the disease remains obscure. At the same time they hoped that further genetic studies would contribute to the clarification of the situation. Using the advanced technique of metabolomics, Struja et al.[73] found no distinct pattern that could relate to disease relapse in cases of Grave’s disease. The same researchers tried to find markers for the early diagnosis of thyroid disease on the basis of metabolomics. The authors could not find any metabolomics parameter that was able to fulfill this expectation[74].
At the time Precision Medicine arrived, Galofre and Ladenson added some clinical features and described some expectations for thyroid diseases in 2016. Galofre wrote: “The aim of the initiative is to eradicate imprecision in estimating the probability of a correct diagnosis, to be as sure as possible of the most effective treatment, and to maximize the chances of a successful outcome”[75]. Ladenson commented: “The broad spectrum of thyroid disease severity - from subclinical hypothyroidism to myxedema coma, subclinical thyrotoxicosis to thyroid storm, and microscopic papillary to anaplastic cancers - has always demanded that clinicians individualize their management of thyroid patients. Deepening knowledge of thyroid pathophysiology along with advances in diagnostic, prognostic, and therapeutic technologies applicable to thyroid diseases position this field to ride the wave of precision medicine in the decade ahead”[76].
Another source of general information on genetics and thyroid disease is the “Genetics Home Reference: your guide to understanding genetic conditions”(https://ghr.nlm.nih.gov/). The interested reader will find the following description of the inheritance of Hashimoto’s disease: “The inheritance pattern of Hashimoto thyroiditis is unclear because many genetic and environmental factors appear to be involved”(https://ghr.nlm.nih.gov/condition/hashimoto-thyroiditis#inheritance). For Graves’ disease, they state the following: “The inheritance pattern of Graves’ disease is unclear because many genetic and environmental factors appear to be involved. However, the condition can cluster in families” (https://ghr.nlm.nih.gov/condition/graves-disease).
Going through the literature, we got the impression that one can consider genetic studies done in relation to thyroiditis to fall into 2 groups: descriptive and interpretative. The first group tells the reader that there are defects somewhere, while the second group goes beyond to describe cellular mechanisms.
Among studies that we consider to be descriptive, one can find mutations in patients with thyroid disease. Genetic changes in Hashimoto’s thyroiditis involving thyroglobulin gene polymorphisms were described by Caputo et al.[77], showing that the TGrl29 microsatellite was significantly associated with the disease. Lo et al.[78] published the results of a study conducted in a family that presented early onset of Hashimoto’s thyroiditis. They found a splice site variant in all family members who were affected, but also in one child without the disease. The change they found was TG c.1076-1G > C. In a setting of genetic polymorphism, i.e., TG rs2076740, the anti-thyroglobulin antibody titers were described as being higher than in controls[79]. In a study that looked at a group of Chinese Han subjects, two independent SNPs (rs2294025 and rs7005834) for Graves’ disease susceptibility were described. Unfortunately, they showed no correlation with clinical phenotypes[80].
Analyses of naturally occurring genetic defects in congenital goiter have concluded that allelic alterations of the thyroglobulin gene lead to a defect in secretion in the sense of an endoplasmic reticulum storage disease[81]. Retention of thyroglobulin, or in general of any protein, will lead to a response via the endoplasmic reticulum in the sense of the unfolded protein response[82,83]. This process requires sufficient ATP supply[84]. In 3 of their publications, Barishev et al.[85,86] Sargsyan et al.[87] described the mechanisms involved in congenital hypothyroidism. Pardo et al.[88] reported that genetic changes in Tg, i.e., the p.A2215D thyroglobulin gene mutation, can produce molecule retention in cells, thus involving the endoplasmic reticulum. Using a bioinformatics analysis approach, Zheng et al.[89] recently identified key genes associated with Hashimoto’s disease. Another player in Hashimoto’s thyroiditis involves polymorphisms of the STAT3 gene[90,91].
A new publication on genetics has shown that the apoptosis antagonizing transcription factor (AATF)[92] is involved in thyroid disease in determining the volume of the gland in situations of thyroiditis[93]. AATF appears to regulate the anti-apoptotic effect of the unfolded protein response through transcriptional regulation of AKT1[94].
We can summarize this section by declaring that on the basis of the data presented on autoimmunity, selenium supplementation and genetics, it is not possible to develop any therapy concept that can improve or cure benign thyroid disease. The following Box 2 is Bob Dylan quotation from his 1967 song “All Along the Watchtower” describes the situation precisely.
To provide relief, we will present our approach to diagnosing and treating thyroid disease based on our WOMED model of benign thyroid disease[8,9].
Finding relief for thyroid disease: correlative observations of thyroid perfusion changes
(1) The Index Case: combined deficiencies of magnesium, selenium and CoQ10
The Index Case of a complex biochemical and clinical situation involved a young woman who was examined between 2008 and 2020. The patient presented with a series of sequential medical conditions including hyperthyroidism, cerebral ischemia, and myocardial infarction. The biochemical determinations detected low levels of magnesium and CoQ10. The first thyroid sonography was done in May 2008 and showed a hypo-echogenic gland with massive hyperperfusion as seen in color mode. Supplementation with magnesium and CoQ10 normalized thyroid morphology and function. In 2018, a postpartum thyroiditis with hyperthyroidism and marked magnesium deficiency was diagnosed. On ultrasound, the thyroid showed a pronounced diminution of echogenicity together with hyperperfusion suggestive of CoQ10 deficiency. Since the patient was breastfeeding, a combined supplementation was started again using a higher daily dose of magnesium, i.e., 11.2 mmol of elemental magnesium (~3.2 g magnesium citrate). CoQ10 was given at a dose of 30 mg/d. Within 3 months, thyroid function, thyroid morphology and perfusion returned to normal. During her first pregnancy, hyperperfusion with the pattern of CoQ10 deficiency reappeared. Currently, in the second pregnancy, the changes were prevented by continuing supplementation [Figure 1].
Figure 1. The Index Case showing combined deficiencies of magnesium, selenium, and CoQ10. Thyroid sonography was done using 2 different systems: SIEMENS Antares in 2008 (A, B) and GE LOGIQ E9 in 2019 (C, D). In 2009, the thyroid showed diminished echogenicity and hyperperfusion as seen in color mode. The images C and D demonstrate the improvement of thyroid morphology together with a normalization of perfusion. These changes disappeared after combined supplementation was started. Currently the patient does not require any thyroid medication
(2) Thyroid perfusion changes during pregnancy
One pregnant woman seen between April and August 2019 showed sequentially appearing patterns of altered thyroid perfusion: (1) magnesium deficiency; and (2) CoQ10 deficiency [Figure 2].
Figure 2. Changes in thyroid perfusion during pregnancy in the same patient: the characteristic spot-like hyperperfusion due to magnesium deficiency on the 25th week of pregnancy (A); the situation 10 weeks later with the typical vessel form seen in CoQ10 deficiency, i.e., vessels with a wide appearance (B). Thyroid function was normal at both examinations
(3) Hyperthyroidism developing after combined heat and exertional stress
In the past 2 years, we observed a small number of hyperthyroid patients who initially presented with signs and symptoms suggestive of cardiac disease. Laboratory tests showed an elevation of NT-pro-BNP and creatine kinase. Echocardiography did not reveal cardiomyopathy. In one male patient, heat stress together with intense physical activity (active sports vacation) was the precipitating event for hyperthyroidism [Figure 3]. The patient complained of reduced physical capacity almost to the level of physical exhaustion, while the time of physical recovery was prolonged. This situation corresponds to the picture seen in heatstroke when there has been vigorous muscle exertion[95].
Figure 3. Hyperthyroidism after exertional stress under conditions of elevated ambient temperature. Initial examination showing diffusely increased vascularization (A); in the follow-up examination after 9 months of combined supplementation, hyperperfusion is less pronounced while the thyroid still shows decreased echogenicity (B). The patient is currently euthyroid after 2 years
(4) Postpartum thyroiditis and hyperthyroidism
The sibling of the previous patient [Figure 4] presented with a condition of postpartum thyroiditis with hyperthyroidism, showing less pronounced changes in thyroid morphology. Signs of CoQ10-associated hyperperfusion were present. She responded well to supplementation within 3 months. Thyroid morphology and function returned to normal in 2020.
Figure 4. Postpartum thyroiditis with hyperthyroidism. The thyroid is hypo-echogenic. The perfusion pattern corresponds to a combined deficiency of magnesium (small spots) and CoQ10 (wide vessels)
(5) Hyperthyroidism and elevated proBNP
A recent case presented with symptoms of decreased muscular function, i.e., fatigability. Laboratory tests demonstrated a T3-predominant hyperthyroidism with > 30.8 pmol/L fT3, 64.2 pmol/L fT4 together with elevated proBNP of 811 pg/ml. Combined supplementation with magnesium citrate (4 g daily), 60 mg CoQ10, and 200 µg selenomethione was commenced together with thyreostatic treatment. A follow-up 3 weeks later showed significant decrease of thyroid hormone levels and normalization of proBNP. Thyroid hyperperfusion was also diminished [Figure 5].
Figure 5. Recently diagnosed case of hyperthyroidism showing hyperperfusion corresponding to CoQ10 deficiency. Sonography images are compared for 2 different machines, i.e., Toshiba (now Canon) (A, B) and Loqig Q9, G.E (C, D). The scintigraphy shows an intense tracer uptake. The lower right panel shows the response to 3 weeks of combined supplementation
(6) Chronic relapsing thyroiditis: 3D power Doppler and 18F-FDG PET investigation
The first case of thyroiditis that could be investigated some 15 years ago and showed a course of repeated relapses of the thyroid affection. Every time a relapse occurred, hyperperfusion of the thyroid with the pattern of CoQ10 deficiency reappeared. In July 2019 a whole body 18F-FDG PET examination was requested by the patient’s general practitioner. The study revealed that the metabolism of the thyroid and the heart had switched to glycolysis. The patient had no signs of heart disease [Figure 6].
Figure 6. Woman with chronic relapsing thyroiditis, showing increased uptake of 18F-FDG in the thyroid and the heart in a PET examination (A). This tracer uptake pattern indicates glycolytic metabolism of the organs. On 3D power Doppler sonography (B), one can recognize the marked perfusion changes that correspond to CoQ10 deficiency, showing enlarged vessels
Correlative observations on blood levels of magnesium and anti-thyroglobulin antibodies
The following images are an example of the effects of combined supplementation on the levels of anti-thyroglobulin antibodies and magnesium in a female patient with hyperthyroidism. At the same time as magnesium levels tended to normalize, i.e., > 0.80 mmol/L, anti-thyroglobulin antibodies decreased into the normal range [Figure 7]. Fluctuations in magnesium levels were due to compliance failures.
Figure 7. The graphs show the sequential determinations of magnesium levels (A) and anti-thyroglobulin antibody levels (B) documented between 2017 and 2020
The corresponding ultrasound images of this patient are shown in Figures 8 and 9. Thyroid volume decreases and morphology improves, while perfusion normalizes.
Figure 9. Power Doppler sonography showing a diminution of perfusion September 2017 to January 2020. Thyroid size decreases, morphology improves and perfusion returns to normal
We (Moncayo R and Reisenzahn J) are currently analyzing the relationships between anti-thyroglobulin antibodies and magnesium. A rough estimate shows a good response rate of about 80%, partial improvement in 10%, and no improvement in the remaining 10% of cases. The non-responding cases fall into the category of fibrosis, where only replacement therapy with thyroid hormone can be offered. Some biochemical and genetic features of thyroglobulin will be discussed below.
Modern ultrasound and biochemistry parameters depict the finding made by Hakaru Hashimoto
On the basis of the data presented above it is possible to put together a functional model of thyroid disease pathogenesis centered on thyroiditis, where discrete biochemical players assume a specific role in the process of thyroid damage. The tool needed to recognize these changes is power Doppler sonography done with a modern probe of at least 15 MHz frequency[7]. In each patient, it is possible to differentiate distinct phases of thyroid inflammation and consequently to prescribe a tailored supplementation. Follow-up controls rely on 3 elements: (1) clinical history; (2) ultrasound examination with power Doppler; and (3) laboratory determinations of thyroid hormones, thyroid antibodies and magnesium. In some cases, ferritin and vitamin D are also evaluated. Within a time period of 1-2 years, normalization of parenchymal and perfusion changes can be seen[9,10]. The following Figure 10 shows our practical clinical approach[7-10] as compared to the classical description made by Hashimoto[14].
Figure 10. Summary of the WOMED clinical approach to thyroiditis in relation to the 3 main findings described by Hashimoto (in the original German description). The clinical validity of diagnostic procedures including power Doppler sonography is explained for every stage
The findings of the sonography examinations can be translated into clinical action, i.e., into a therapeutic approach [Table 2]. Since sonography is regularly excluded from clinical practice recommendations, we cannot compare our approach with any previous publications.
Interpretation matrix of 3D power Doppler thyroid sonography
| Morphology | Perfusion | Magnesium | Coenzyme Q10 | Selenium |
|---|---|---|---|---|
| Normal | Normal | |||
| Hypo-echogenic homogeneous | Fine granular | Deficiency | ||
| Hypo-echogenic inhomogeneous | Thickened vessels | Deficiency | Deficiency | |
| Hypo-echogenic inhomogeneous with fibrosis signs | Moderately increased | Probable | Probable | Deficiency |
| Very low echogenic | Increased | Deficiency | Deficiency | Deficiency |
| Very low echogenic with fibrosis signs | Not increased = final stage | - | - | - |
Clinical practice has led us to the following prescription recommendations. Magnesium deficiency should be treated with pure magnesium citrate as a magistral formulation at a dose of 3.5 to 4.0 g per day. This applies to hypothyroidism as well as to hyperthyroidism. The main advantage of this formulation lies in the ability of citrate to localize inflammation[96]. For the correction of selenium and CoQ10 deficiencies, we have relied on products from Pure Encapsulations for 15 years and have obtained satisfactory results [Figures 1-9, and Figure 11]. The preparations used include selenomethionine (200 µg capsule) and CoQ10 (60 mg capsule). Supplementation begins with 1 capsule of selenomethionine and CoQ10 daily taken together at night during the first 2 weeks. Beginning on the 3rd week the dose is reduced to only 3 capsules per week of each preparation taken on alternate days. Treatment costs amount to €220 per year or €0.66 per day. The target values to be reached through supplementation are shown in Figure 11. The figure suggests an interlacing of the substances in human physiology. The essence of such an approach can be taken from the abstract of the publication by Kitano on Systems biology: “To understand biology at the system level, we must examine the structure and dynamics of cellular and organismal function, rather than the characteristics of isolated parts of a cell or organism”[97]. This interlacing is shown in Figure 12.
Figure 11. The target concentrations to be reached following supplementation. The graphic represents the interlaced function of the individual biochemical elements. Sequential ultrasound controls showing morphology (upper panel) and power Doppler perfusion (lower panel) September 2017 to January 2020. Thyroid size decreases, morphology improves and perfusion returns to normal
Figure 12. The WOMED concept of Shared Resources. The central element is defined by sufficient supply of magnesium, iron, selenium, and CoQ10. This common source will allow normal mitochondrial function. Sufficient ATP production will have positive effects on the function of the endoplasmic reticulum
We have observed that heat stress can affect levels of CoQ10 and initiate or complicate the course of thyroid disease. Israeli authors recognized 40 years ago that intermittent thyroid dysfunction corresponded to a heat stress syndrome[98]. Under experimental conditions, CoQ10 can ameliorate the consequences of heat stress in chickens[99]. For thyroid disease patients planning a holiday where they will be exposed to elevated temperatures, we recommend CoQ10 daily[100]. Theoretically the same applies to UV-radiation[101,102], which also affects CoQ10. Physical and psychological stressors can have a negative effect on magnesium, selenium and CoQ10. Loss of CoQ10 could affect oxidative phosphorylation in muscle in such a manner that energy production will rely on glycolysis. This has been described in brown adipose tissue[103]. The inverse situation can be seen in the regulation of muscle oxidative function away from glycolysis achieved by CoQ10[104].
The role of thyroglobulin conformation: isolation methods and biochemistry to genetics related to the endoplasmic reticulum
The biochemical methods for the isolation of thyroglobulin have been developed over the past 120 years, contributing with information about molecular variability in the thyroid. An early description of the isolation of thyroglobulin was provided by Oswald in 1898[105] while working at the Chemical Laboratory of the Medical Clinic in Zürich. The method was based on the extraction of the thyroid glands with 0.9% NaCl, followed by precipitation by either half saturation with ammonium sulfate or with acetic acid. In 1933, Heidelberg and Palmer commented that Oswald’s method was to be considered as a reference work even though it produced denaturation of the molecule and it also contained some contaminants[106]. In the following years, Heidelberger and Svedberg determined the molecular weight of thyroglobulin[107], and Heidelberger and Pedersen determined the isoelectric point of the molecule[108].
In 1948, Derrien, Michel and Roche published their research work dealing with the isolation of thyroglobulin based on a salting-out procedure[109]. A short discussion on the role of thyroglobulin in thyroid disease was published by Witebsky in 1958[110]. In that study, thyroglobulin was extracted by sedimentation with 1.6 and 1.7 M ammonium sulfate. The authors suggested that thyroglobulin was the agent leading to a serological reaction. In 1968, Cheng et al.[111] described an extraction method that combined sequential salting-out with ammonium sulfate, starch-gel electrophoresis, and gel filtration. They concluded that this procedure allowed them to purify thyroglobulin, eliminating contaminants.
In 1998, Druetta[112] compared the molecular form of thyroglobulin in patients with Graves’ disease (n = 21) and compared these results with the findings of anti-thyroglobulin antibodies. Ten patients were positive for anti-thyroglobulin antibodies. Thyroglobulin was extracted using an 8 to 25% sucrose gradient, followed by centrifugation at 100,000 g for 18 h at 4 °C. In patients with Graves’ disease and positive for anti-thyroglobulin antibodies, the main form of thyroglobulin was found to be a heavy sedimentation peak, i.e., faster than the normal 19S form. Split forms of thyroglobulin were not associated with the presence of antibodies. In subacute thyroiditis, they described small oligomeric forms.
Besides the traditional thought of thyroglobulin being only related to thyroid hormone synthesis, a new perspective of thyroglobulin in the regulation of thyroid function has been proposed by Sellitti and Suzuki[113]. In this model, thyroglobulin is responsible for heterogeneity of follicular cells in which colloidal concentration of thyroglobulin adapts to improve hormone production. They consider the thyroid follicle to be the morphological function unit of the gland having a basket-like surrounding capillary network. Differences in the content of thyroglobulin can be found in adjacent follicles. Gérard et al.[114] showed a process where thyrocytes metabolize thyroglobulin globules by step-wise fragmentation changing the thyroglobulin form from being insoluble compact into soluble molecules. Morphological studies of the thyroid done by Smeds and Anderberg showed that administration of propylthiouracil was associated with a decrease of thyroglobulin aggregates[115]. Smeds[116] also noted that thyroglobulin aggregates localized preferentially to the periphery of the colloid in close association with the apical portion of the thyroid cell membrane.
During the process of thyroglobulin synthesis, a transient aggregation of nascent thyroglobulin in the endoplasmic reticulum can be observed[117]. Thyroglobulin production is regulated by TSH, and at the same time, TSH produces a regulated increase in folding capacity[118]. The 1996 study by Medeiros-Neto et al.[45] dealing with congenital hypothyroidism, demonstrated that these patients suffer from an endoplasmic reticulum storage disease. Electron microscopy clearly showed an abnormal distension of the endoplasmic reticulum. Using biochemical methods they found a strong induction of chaperones such as binding immunoglobulin protein (BiP), a hsp70 homolog, and glucose-related protein 94 (GRP94) in an animal model of congenital goiter, the cog/cog mouse[119,120]. These results indicate an activation of the endoplasmic reticulum[121]. The GRP were originally described in 1977 by Shiu, Pouyssegur, and Pastan who worked with Rous sarcoma virus-transformed chick embryo fibroblasts[122]. They elucidated that the stimulus for GRP induction was glucose depletion in the growth medium. In 1981, Lee[123] added a functional relationship of thermal stress to the induction of these proteins. Thermal stress is related to the heat shock reaction, originally described by Ritossa[42,124].
In some patients with congenital goiter, anti-thyroglobulin antibodies can be found in the blood. Ordookhani et al.[125] found an association between neonatal hypothyroidism and elevated levels of anti-thyroglobulin antibodies. Cho et al.[126] described high titers of anti-thyroglobulin antibodies in cases of non-transient congenital hypothyroidism. They proposed that the antigenicity of thyroid proteins could be related to the underlying pathogenesis of congenital goiter. Peteiro-Gonzalez et al.[127] characterized changes in thyroglobulin genes in a family with congenital goiter, thus confirming their relation to altered function of the endoplasmic reticulum. A general investigation on genetic changes found in cases of congenital goiter was presented by Nicholas et al.[128] in 2016. A specific mutation affecting the linker domain of thyroglobulin, i.e., p.L571P, has been recently described in relation to the conformational maturation and intracellular handling of rat thyroglobulin leading to intracellular retention[129]. An extensive description of the genetics and biochemistry of thyroglobulin was recently published by Citterio et al.[130].
Cell repair, programmed cell death, the unfolded protein response and the heat shock response, and ROS
Cell repair mechanisms have seen an evolution in the terminology used to describe them. There has been the Milieu Intérieur of Claude Bernard[131], homeostasis from Cannon[132], and proteostasis from Balch[133], just to name a few. Beyond internal equilibrium, these processes are now related to chronic modern day diseases, where homeostasis has failed[134]. Susan Elmore published a review on the topic of programmed cell death in 2007[135]. Referring to apoptosis, she reminds the readers that this process is energy-dependent, while on the other hand, necrosis is not. It follows that it is conceivable that transition from apoptosis to necrosis could occur when energy supply is limited, i.e., conservation of ATP production is essential for life. Prior to cell death, other cellular repair mechanisms are activated. Altered molecular structures, i.e., unfolded proteins, can induce a repair mechanism[83].
Looking at thyroid disease, the conformational changes of thyroglobulin, either due to a genetic cause or found in extraction procedures, present a challenge for the endoplasmic reticulum. The endoplasmic reticulum must clear misfolded proteins by activating repair mechanisms[136]. The endoplasmic reticulum relies on ATP translocation to carry out its functions[137]. Furthermore, the activation of the unfolded protein response and the degradation of misfolded proteins involves the ubiquitin system[138] and the 26S proteasome. Stabilization of the 26S proteasome is both ATP- and NADH-dependent[139]. ATP can add a further contribution to structural maintenance since it has important functions as a hydrophore, maintaining protein solubility[140,141].
On the basis of these modern concepts, it is also possible to add an explanation to some experimental observations. Referring to the obese chicken model of thyroid disease, the authors mentioned two mechanisms that seemed to increase disease severity when the animals were kept in crowded cages: temperature and stress. The authors called this condition the “cage effect”, which, however, was not further characterized. Current studies about a similar situation of heat exposure have demonstrated that the process of heat shock reaction is taking place[142] resulting in genomic changes that are associated with outcome survival conditions. Another relevant situation in “the cage” is that of stress since physical stressors can lead to a deficiency condition for magnesium[143] and CoQ10 (oral presentation by Gvozdjáková A. in 2000 cited by Boreková[144]). Low magnesium levels are related to decreased thyroid function[145]. In Figure 3, we demonstrated the effect of heat exposure on CoQ10 levels in relation to thyroid disease as well as the positive corrective action. Similar observations on the protective action of administration of CoQ10 to chickens under heat stress are known in the literature[146,147]. The addition of acetyl salicylic acid to CoQ10 has an enhancing effect on the expression of hsp70 in the chicken heat stress model[148]. Modern studies have demonstrated that exogenous aspirin can activate the signaling pathways of the unfolded protein response[149]. Further information on aspirin and the endoplasmic reticulum can be found in publications dealing with plant physiology[150,151]. We can now say that the empiric use of aspirin to treat patients with thyroiditis as described in 1902 by de Quervain[12] was on the right track and simply ahead of its time.
Besides heat shock and unfolded protein situations, oxidative damage caused by radicals has been investigated in relation to aging and disease for many decades. Early studies on this topic were presented by Gerschman in 1953 (published in 1954[152]). She discussed common mechanisms found in oxygen poisoning and x-irradiation. In 1956, Harman presented his theory of aging, where he considered the deleterious effect arising from cellular metabolism[153]. He compared these effects to those resulting from irradiation in relation to OH and HO2 radicals. In addition to external sources of reactive oxygen species, disruption of complex I of the oxidative phosphorylation chain can turn to be noxious as has been described by Adam-Vizi and Chinopoulos[154]. Another peculiarity of CoQ10 to keep in mind is its tendency towards lower levels with increasing age (table 3, page 582 in[155]).
In a previous section, we discussed the lack of effect of selenium supplementation in thyroid disease when only putative antioxidant effects are sought. Our working model has a different philosophy. Our decision to consider selenium and CoQ10 in our supplementation prescription is based on observations made by Green et al.[156] in 1961 and by Hidiroglou et al.[157] in 1967. Both groups revealed a functional relation between both substances such that administration of selenium improved the tissue levels of CoQ10 in the experimental animals. This important physiological aspect has not been considered in many studies that have chosen to study the effects selenium or CoQ10 given as single agents to overcome oxidative changes. A similar observation on this relation was published by Vadhanavikit and Ganther[158]. In a further study, they speculated that the lowering of CoQ10 levels depended on the depressed GSH-Px activity that resulted from selenium deficiency[159]. In situations of selenium deficiency, mitochondrial structure and also electron transport function were found to be altered[69]. A study on the distribution of selenium in mitochondria revealed that the highest concentration was found in the inner membrane space, the inner membrane and the matrix[160]. Finally, we would like to mention that selenium and the endoplasmic reticulum are a matter of current research related to resident selenoproteins. Details can be found in the references[161-169].
A concept of shared resources as a general basis of health and disease
The medical literature contains a considerable number of publications that suggest that thyroid function has effects on other body systems, e.g., fertility and reproduction. We would like to address this topic adding a mechanism that we refer to as the WOMED Concept of Shared Resources. The basic interaction of magnesium, selenium, CoQ10 and iron in relation to thyroid function is shown in Figure 13. What we initially conceived for thyroid function has applicability in the whole body. Iron has a high relevance since it is a constituent in countless iron-sulfur proteins[170-178].
Figure 13. The combined availability of magnesium, iron, selenium, and CoQ10 will maintain key functions in the body, i.e., mitochondria
The WOMED Concept of Shared Resources is shown in Figure 12. The central condition is defined by sufficient supply of magnesium, iron, selenium, and CoQ10. B. This common source will allow normal mitochondrial function and consequently ensure the function of the endoplasmic reticulum, i.e., protein repair. Lack of CoQ10 affects primarily energy generation and is related to multi-organ involvement[179]. The graphic represents Resource Distribution as a flow continuum inspired by the movement of Qi in Chinese Medicine[180-191] and taking into consideration the idea of metabolic flexibility[192].
Looking back and beyond, we now consider thyroid disease as a model of acquired unfolded protein disease, thus proposing that the concepts of thyroid physiology should be modified. In the final phases of writing this manuscript, we found a recent contribution from the research group of Peter Arvan describing the role of endoplasmic reticulum stress on the survival and adaptation of thyrocytes[193]. We believe that magnesium plays an important role in this process of adaptation by being a key element in the generation of ATP, which in turn results in the recovery of the function of the endoplasmic reticulum. In other words, low levels of magnesium are associated with misfolded thyroglobulin molecules, which induce the production of anti-thyroglobulin antibodies. The risk of developing anti-thyroglobulin antibodies in situations of magnesium deficiency was described by Wang in 2018[50]. Our data confirm and expand these previous findings. In 2019, Ana Barić described an association between symptom burden and anti-thyroglobulin antibodies in patients with Hashimoto’s thyroiditis[49]. These findings coincide with our previous description of the psychosomatic aspects of thyroid disease, where we found an association between magnesium deficiency and symptoms[194]. In 2020, Luka Brčić together with Ana Barić and others reported that the expression of AATF is diminished in cases of Hashimoto’s thyroiditis and is associated with increased thyroid volume[93]. In the historical and experimental sections of this paper, we mentioned that increased thyroid volume has been a characteristic known for decades.
The important role of genetics in this context can be recognized in recent studies by Santos et al.[195], demonstrating a relation between the SELENOS promoter genotype and NFE2L2 promoter genotype, which together modulate the risk of Hashimoto’s thyroiditis. SELENOS is of importance since it constitutes a basic element of the endoplasmic reticulum, especially under conditions of glycolytic metabolism[196]. In Figure 6, we indeed demonstrated that chronic thyroiditis in an active phase can be associated with glycolytic metabolism of the thyroid.
Since the elements leading to this acquired situation are amenable to correction, the fears related to the artificial concept of autoimmunity should disappear from the minds of patients and medical practitioners. More emphasis should be put on maintaining a balanced state of the nutritional elements that the body must share - magnesium, selenium, iron and CoQ10.
Declarations
AcknowledgmentsThe principal author (Moncayo R) was introduced to thyroid research by Rodrigo Fierro-Benítez (Quito, Ecuador, 1976-1979). At this time, the teachings of Gregorio Marañon[197,198] transmitted by Fierro-Benítez were also absorbed. This period was followed by valuable scientific exchange with John B. Stanbury (Boston), John Dunn (Charlottesville) and Georg Riccabona (Innsbruck) in the context of the iodine deficiency network[199-202]. Many important and contemporary concepts and lessons have been found in the publications from Gerardo Medeiros-Neto (São Paulo), Peter Arvan (Ann Arbor), and Carina M. Rivolta, Cintia E. Citterio, and Héctor M. Targovnik (Buenos Aires).
Authors’ contributionsMain concept for the manuscript, literature search, preparing the figures, writing the manuscript: Moncayo R
Developed the method of power Doppler sonography for thyroid patients, financial support: Moncayo H
Extracted clinical data from patients shown in the Figures, literature search on selenium and thyroid, reading the publications and extracting the content: Reisenzahn J
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateAll procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This paper was written in the form of a case report, retrospectively describing the course of the diagnostics and therapy. The concept was considered and approved by the Medical University of Innsbruck (Studienabteilung).
Consent for publicationInformed consent was obtained from the patient for the publication of this report.
Copyright© The Author(s) 2020.
REFERENCES
1. Zárate A. Gregorio Marañon, un pionero de la endocrinología, cumple 50 años de su fallecimiento. Gaceta médica de México 2011;147:176-9.
2. Szent-Györgyi A. Bioenergetics. New York: Academic Press; 1957.
3. Jameson JL, Longo DL. Precision medicine--personalized, problematic, and promising. N Engl J Med 2015;372:2229-34.
4. Danziger L, Elmergreen GL. The thyroid-pituitary homeostatic mechanism. Bull Math Biophys 1956;18:1-13.
5. Bekkering GE, Agoritsas T, Lytvyn L, Heen AF, Feller M, et al. Thyroid hormones treatment for subclinical hypothyroidism: a clinical practice guideline. BMJ 2019;365:l2006.
6. Taylor P, Bianco AC. Urgent need for further research in subclinical hypothyroidism. Nat Rev Endocrinol 2019;15:503-4.
7. Moncayo R, Moncayo H. Advanced 3D sonography of the thyroid: focus on vascularity. Sonography. Rijeka, Croatia: Intech; 2012. pp. 273-92.
8. Moncayo R, Moncayo H. Applying a systems approach to thyroid physiology: looking at the whole with a mitochondrial perspective instead of just TSH values or why we should know more about mitochondria to understand metabolism. BBA Clin 2017;7:127-40.
9. Moncayo R, Moncayo H. The WOMED model of benign thyroid disease: acquired magnesium deficiency due to physical and psychological stressors relates to dysfunction of oxidative phosphorylation. BBA Clin 2015;3:44-64.
10. Moncayo R, Moncayo H. Proof of concept of the WOMED model of benign thyroid disease: Restitution of thyroid morphology after correction of physical and psychological stressors and magnesium supplementation. BBA Clin 2015;3:113-22.
11. Riedel BM. Die chronische, zur Bildung eisenharter Tumoren führende Entzündung der Schilddrüse. Verh Dtsch Ges Chir 1896;25:101-5.
12. de Quervain F. Ueber acute, nicht eiterige Thyroiditis. Arch f klin Chir 1902;67:706-14.
13. McCarrison R. Observations on endemic cretinism in the chitral and gilgit valleys. Ind Med Gaz 1908;43:441-9.
14. Hashimoto H. Zur Kenntniss der lymphomatösen Veränderung der Schilddrüse (Struma lymphomatosa). Arch Klin Chir 1912;97:219-48.
15. Graham A, McCullagh EP. Atrophy and fibrosis associated with lymphoid tissue in the thyroid - Struma lymphomatosa (Hashimoto). Arch Surg 1931;22:548-67.
16. Graham A. Riedel’s struma in contrast to struma lymphomatosa. West J Surg 1931;39:681-92.
17. Eisen D. The Relationship between Riedel’s Struma and Struma Lymphomatosa (Hashimoto). Can Med Assoc J 1934;31:147-50.
18. Clute HM, Eckerson EB, Warren S. Clinical aspects of struma lymphomatosa (Hashimoto). Arch Surg 1935;31:419-28.
19. McClintock JC, Wright AW. Riedel’s struma and struma lymphomatosa (Hashimoto): a comparative study. Ann Surg 1937;106:11-32.
21. Lundbæk K. On chronic Thyroiditis, Riedel’s Struma, »Hashimoto’s Struma», and Lymphadenoid goiter, with Report of a Case of Chronic Thyroiditis. Acta Medica Scandinavica 1942;112:55-67.
22. Simmonds M. Über chronische Thyreoiditis und fibröse Atrophie der Thyreoidea. Virchows Archiv Fur Pathologische Anatomie Und Physiologie Und Fur Klinische Medizin 1923;246:140-50.
24. Statland H, Wasserman MM, Vickery AL. Struma lymphomatosa (Hashimoto’s struma); review of fifty-one cases with a discussion of the endocrinologic aspects. AMA Arch Intern Med 1951;88:659-78.
25. Witebsky E, Rose NR, Terplan K, Paine JR, Egan RW. Chronic thyroiditis and autoimmunisation. JAMA 1957;164:1439-47.
26. Roitt IM, Doniach D, Campbell PN, Hudson RV. Auto-antibodies in Hashimoto’s disease (lymphadenoid goitre). Lancet 1956;271:820-1.
27. Doniach D, Roitt IM. Autoimmunity in Hashimoto’s disease and its implications. J Clin Endocrinol Metab 1957;27:1293-304.
28. Burek CL. Autoimmune thyroiditis research at Johns Hopkins University. Immunol Res 2010;47:207-15.
30. Katz SM, Vickery AL Jr. The fibrous variant of Hashimoto’s thyroiditis. Hum Pathol 1974;5:161-70.
31. McCarrison R. Note on the experimental production of lymphadenoid goitre in rats. Br Med J 1929;1:5-6.
32. Freund J, Casals J, Page Hosmer E. Sensitization and antibody formation after injection of tubercle bacilli and paraffin oil. Exp Biol Med 1937;37:509-13.
33. Shulman S, Rose NR, Witebsky E. Studies on organ specificity. III. Ultracentrifugal and electrophoretic examinations of thyroid extracts. J Immunol 1955;75:291-300.
34. Weigle WO. The induction of autoimmunity in rabbits following injection of heterologous or altered homologous thyroglobulin. J Exp Med 1965;121:289-308.
37. Leo O, Cunningham A, Stern PH. Vaccine immunology. Perspect Vaccinol 2011;1:25-59.
38. Caturegli P, De Remigis A, Rose NR. Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmun Rev 2014;13:391-7.
39. Rose NR, Witebsky E. Studies on organ specificity. II. Serological interrelationships among thyroid extracts of various species. J Immunol 1955;75:282-90.
40. Witebsky E, Rose NR, Shulman S. Studies on organ specificity. I. The serological specificity of thyroid extracts. J Immunol 1955;75:269-81.
41. Roitt IM, Campbell PN, Doniach D. The nature of the thyroid auto-antibodies present in patients with Hashimoto’s thyroiditis (lymphadenoid goitre). Biochem J 1958;69:248-56.
42. Ritossa F. A new puffing pattern induced by temperature shock and DNP in drosophila. Experientia 1962;18:571-3.
43. Wartenberg J, Doniach D, Brostoff J, Roitt IM. Leucocyte migration inhibition with mitochondria in human autoimmune thyroid disorders. Clin Exp Immunol 1973;14:203-12.
44. White GH, Walmsley RN. Plasma enzyme activities in primary hypothyroidism. Clin Chem 1984;30:323-5.
45. Medeiros-Neto G, Kim PS, Yoo SE, Vono J, Targovnik HM, et al. Congenital hypothyroid goiter with deficient thyroglobulin. Identification of an endoplasmic reticulum storage disease with induction of molecular chaperones. J Clin Invest 1996;98:2838-44.
47. Zimmermann FA, Neureiter D, Feichtinger RG, Trost A, Sperl W, et al. Deficiency of respiratory chain complex I in Hashimoto thyroiditis. Mitochondrion 2016;26:1-6.
48. Grootjans J, Kaser A, Kaufman RJ, Blumberg RS. The unfolded protein response in immunity and inflammation. Nat Rev Immunol 2016;16:469-84.
49. Barić A, Brčić L, Gračan S, Škrabić V, Brekalo M, et al. Thyroglobulin antibodies are associated with symptom burden in patients with Hashimoto’s thyroiditis: a cross-sectional study. Immunol Invest 2019;48:1-12.
50. Wang K, Wei H, Zhang W, Li Z, Ding L, et al. Severely low serum magnesium is associated with increased risks of positive anti-thyroglobulin antibody and hypothyroidism: a cross-sectional study. Sci Rep 2018;8:9904.
51. Hu X, Go YM, Jones DP. Omics integration for mitochondria systems biology. Antioxid Redox Signal 2020;32:853-72.
53. van Zuuren EJ, Albusta AY, Fedorowicz Z, Carter B, Pijl H. Selenium supplementation for Hashimoto’s thyroiditis. Cochrane Database Syst Rev 2013:CD010223.
54. Turker O, Kumanlioglu K, Karapolat I, Dogan I. Selenium treatment in autoimmune thyroiditis: 9-month follow-up with variable doses. J Endocrinol 2006;190:151-6.
55. Negro R, Greco G, Mangieri T, Pezzarossa A, Dazzi D, et al. The influence of selenium supplementation on postpartum thyroid status in pregnant women with thyroid peroxidase autoantibodies. J Clin Endocrinol Metab 2007;92:1263-8.
56. Karanikas G, Schuetz M, Kontur S, Duan H, Kommata S, et al. No immunological benefit of selenium in consecutive patients with autoimmune thyroiditis. Thyroid 2008;18:7-12.
57. Krysiak R, Okopien B. The effect of levothyroxine and selenomethionine on lymphocyte and monocyte cytokine release in women with Hashimoto’s thyroiditis. J Clin Endocrinol Metab 2011;96:2206-15.
58. Cochrane AL. Effectiveness and efficiency: random reflections on health services. Nuffield: Nuffield Provincial Hospitals Trust; 1972.
59. Winther KH, Bonnema SJ, Cold F, Debrabant B, Nybo M, et al. Does selenium supplementation affect thyroid function? Results from a randomized, controlled, double-blinded trial in a Danish population. Eur J Endocrinol 2015;172:657-67.
60. Hegedüs L, Bonnema SJ, Winther KH. Selenium in the treatment of thyroid diseases: an element in search of the relevant indications? Eur Thyroid J 2016;5:149-51.
61. Negro R, Attanasio R, Grimaldi F, Marcocci C, Guglielmi R, et al. A 2016 Italian survey about the clinical use of selenium in thyroid disease. Eur Thyroid J 2016;5:164-70.
62. Winther KH, Wichman JE, Bonnema SJ, Hegedüs L. Insufficient documentation for clinical efficacy of selenium supplementation in chronic autoimmune thyroiditis, based on a systematic review and meta-analysis. Endocrine 2017;55:376-85.
63. Wu Q, Rayman MP, Lv H, Schomburg L, Cui B, et al. Low population selenium status is associated with increased prevalence of thyroid disease. J Clin Endocrinol Metab 2015;100:4037-47.
64. Mao J, Pop VJ, Bath SC, Vader HL, Redman CW, et al. Effect of low-dose selenium on thyroid autoimmunity and thyroid function in UK pregnant women with mild-to-moderate iodine deficiency. Eur J Nutr 2016;55:55-61.
65. Esposito D, Rotondi M, Accardo G, Vallone G, Conzo G, et al. Influence of short-term selenium supplementation on the natural course of Hashimoto’s thyroiditis: clinical results of a blinded placebo-controlled randomized prospective trial. J Endocrinol Invest 2017;40:83-9.
66. Neville LF, Mathiak G, Bagasra O. The immunobiology of interferon-gamma inducible protein 10 kD (IP-10): a novel, pleiotropic member of the C-X-C chemokine superfamily. Cytokine Growth Factor Rev 1997;8:207-19.
67. Luster AD, Unkeless JC, Ravetch JV. γ-Interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature 1985;315:672-6.
68. Pirola I, Rotondi M, Cristiano A, Maffezzoni F, Pasquali D, et al. Selenium supplementation in patients with subclinical hypothyroidism affected by autoimmune thyroiditis: results of the SETI study. Endocrinol Diabetes Nutr 2020;67:28-35.
69. Rani P, Lalitha K. Evidence for altered structure and impaired mitochondrial electron transport function in selenium deficiency. Biol Trace Elem Res 1996;51:225-34.
71. Davies TF. Really significant genes for autoimmune thyroid disease do not exist—so how can we predict disease? Thyroid 2007;17:1027-9.
72. Marinò M, Latrofa F, Menconi F, Chiovato L, Vitti P. Role of genetic and non-genetic factors in the etiology of Graves’ disease. J Endocrinol Invest 2015;38:283-94.
73. Struja T, Eckart A, Kutz A, Huber A, Neyer P, et al. Metabolomics for prediction of relapse in Graves’ disease: observational pilot study. Front Endocrinol (Lausanne) 2018;9:623.
74. Struja T, Eckart A, Kutz A, Neyer P, Kraenzlin M, et al. Metabolomics and their ability to distinguish thyroid disorders: a retrospective pilot study. Horm Metab Res 2019;51:256-60.
75. Galofré JC, Díez JJ, Cooper DS. Thyroid dysfunction in the era of precision medicine. Endocrinología y Nutrición (English Edition) 2016;63:354-63.
76. Ladenson PW. Precision medicine comes to thyroidology. J Clin Endocrinol Metab 2016;101:799-803.
77. Caputo M, Rivolta CM, Mories T, Corrales JJ, Galindo P, et al. Analysis of thyroglobulin gene polymorphisms in patients with autoimmune thyroiditis. Endocrine 2010;37:389-95.
78. Lo MS, Towne M, VanNoy GE, Brownstein CA, Lane AA, et al. Monogenic hashimoto thyroiditis associated with a variant in the thyroglobulin (TG) gene. J Autoimmun 2018;86:116-9.
79. Mizuma T, Watanabe M, Inoue N, Arakawa Y, Tomari S, et al. Association of the polymorphisms in the gene encoding thyroglobulin with the development and prognosis of autoimmune thyroid disease. Autoimmunity 2017;50:386-92.
80. Xuan M, Zhao SX, Yan CY, Yang J, Li Y, et al. Fine mapping of thyroglobulin gene identifies two independent risk loci for Graves’ disease in Chinese Han population. Ann Transl Med 2019;7:434.
81. Vono-Toniolo J, Rivolta CM, Targovnik HM, Medeiros-Neto G, Kopp P. Naturally occurring mutations in the thyroglobulin gene. Thyroid 2005;15:1021-33.
82. Oikonomou C, Hendershot LM. Disposing of misfolded ER proteins: a troubled substrate’s way out of the ER. Mol Cell Endocrinol 2019;500:110630.
84. Depaoli MR, Hay JC, Graier WF, Malli R. The enigmatic ATP supply of the endoplasmic reticulum. Biol Rev Camb Philos Soc 2019;94:610-28.
85. Baryshev M, Sargsyan E, Wallin G, Lejnieks A, Furudate S, et al. Unfolded protein response is involved in the pathology of human congenital hypothyroid goiter and rat non-goitrous congenital hypothyroidism. J Mol Endocrinol 2004;32:903-20.
86. Baryshev M, Sargsyan E, Mkrtchian S. ERp29 is an essential endoplasmic reticulum factor regulating secretion of thyroglobulin. Biochem Biophys Res Commun 2006;340:617-24.
87. Sargsyan E, Baryshev M, Szekely L, Sharipo A, Mkrtchian S. Identification of ERp29, an endoplasmic reticulum lumenal protein, as a new member of the thyroglobulin folding complex. J Biol Chem 2002;277:17009-15.
88. Pardo V, Vono-Toniolo J, Rubio IG, Knobel M, Possato RF, et al. The p.A2215D thyroglobulin gene mutation leads to deficient synthesis and secretion of the mutated protein and congenital hypothyroidism with wide phenotype variation. J Clin Endocrinol Metab 2009;94:2938-44.
89. Zheng L, Dou X, Song H, Wang P, Qu W, et al. Bioinformatics analysis of key genes and pathways in Hashimoto thyroiditis tissues. Biosci Rep 2020;40.
90. Kotkowska A, Sewerynek E, Domańska D, Pastuszak-Lewandoska D, Brzeziańska E. Single nucleotide polymorphisms in the STAT3 gene influence AITD susceptibility, thyroid autoantibody levels, and IL6 and IL17 secretion. Cell Mol Biol Lett 2015;20:88-101.
91. Xiao L, Muhali FS, Cai TT, Song RH, Hu R, et al. Association of single-nucleotide polymorphisms in the STAT3 gene with autoimmune thyroid disease in Chinese individuals. Funct Integr Genomics 2013;13:455-61.
92. Page G, Lödige I, Kögel D, Scheidtmann KH. AATF, a novel transcription factor that interacts with Dlk/ZIP kinase and interferes with apoptosis. FEBS Lett 1999;462:187-91.
93. Brčić L, Barić A, Benzon B, Brekalo M, Gračan S, et al. AATF and SMARCA2 are associated with thyroid volume in Hashimoto’s thyroiditis patients. Sci Rep 2020;10:1754.
94. Ishigaki S, Fonseca SG, Oslowski CM, Jurczyk A, Shearstone JR, et al. AATF mediates an antiapoptotic effect of the unfolded protein response through transcriptional regulation of AKT1. Cell Death Differ 2010;17:774-86.
95. Stillman JH. Heat Waves, the new normal: summertime temperature extremes will impact animals, ecosystems, and human communities. Physiology (Bethesda) 2019;34:86-100.
96. Ercan MT, Aras T, Unlenen E, Unlü M, Unsal IS, et al. 99mTc-citrate versus 67Ga-citrate for the scintigraphic visualization of inflammatory lesions. Nucl Med Biol 1993;20:881-7.
98. Sulman FG, Tal E, Pfeifer Y, Superstine E. Intermittent hyperthyreosis -- a heat stress syndrome. Horm Metab Res 1975;7:424-8.
99. Kikusato M, Nakamura K, Mikami Y, Mujahid A, Toyomizu M. The suppressive effect of dietary coenzyme Q10 on mitochondrial reactive oxygen species production and oxidative stress in chickens exposed to heat stress. Anim Sci J 2016;87:1244-51.
100. Schniertshauer D, Muller S, Mayr T, Sonntag T, Gebhard D, et al. Accelerated regeneration of ATP level after irradiation in human skin fibroblasts by Coenzyme Q10. Photochem Photobiol 2016;92:488-94.
101. Trautinger F. Heat shock proteins in the photobiology of human skin. J Photochem Photobiol B 2001;63:70-7.
102. Adair FW. Inhibition of oxygen utilization and destruction of ubiquinone by ultraviolet irradiation of Thiobacillus thiooxidans. J Bacteriol 1968;95:147-51.
103. Anderson CM, Kazantzis M, Wang J, Venkatraman S, Goncalves RL, et al. Dependence of brown adipose tissue function on CD36-mediated coenzyme Q uptake. Cell Rep 2015;10:505-15.
104. Bendahan D, Desnuelle C, Vanuxem D, Confort-Gouny S, Figarella-Branger D, et al. 31P NMR spectroscopy and ergometer exercise test as evidence for muscle oxidative performance improvement with coenzyme Q in mitochondrial myopathies. Neurology 1992;42:1203-8.
106. Heidelberger M, Palmer WW. The preparation and properties of thyroglobulin. J Biol Chem 1933;101:433-9.
108. Heidelberger M, Pedersen KO. The molecular weight and isoelectric point of thyroglobulin. J Gen Physiol 1935;19:95-108.
109. Derrien Y, Michel R, Roche J. Recherches sur la preparation et les proprietes de la thyroglobulin pure I. Biochimica Et Biophysica Acta 1948;2:454-70.
110. Witebsky E, Rose NR, Shulman S. The autoantibody nature of the thyroiditis antibody and the role of thyroglobulin in the reaction. Lancet 1958;1:808-10.
111. Cheng HF, Peterson RE, Evans TC. Preparation of highly purified human thyroglobulin. Biochim Biophys Acta 1968;168:161-4.
112. Druetta L, Croizet K, Bornet H, Rousset B. Analyses of the molecular forms of serum thyroglobulin from patients with Graves’ disease, subacute thyroiditis or differentiated thyroid cancer by velocity sedimentation on sucrose gradient and Western blot. Eur J Endocrinol 1998;139:498-507.
113. Sellitti DF, Suzuki K. Intrinsic regulation of thyroid function by thyroglobulin. Thyroid 2014;24:625-38.
114. Gérard AC, Denef JF, Colin IM, van den Hove MF. Evidence for processing of compact insoluble thyroglobulin globules in relation with follicular cell functional activity in the human and the mouse thyroid. Eur J Endocrinol 2004;150:73-80.
115. Smeds S, Anderberg B. Depletion of colloid 27S and larger thyroid iodoproteins following treatment with propylthiouracil. Acta Endocrinol (Copenh) 1979;91:644-9.
116. Smeds S. On the distribution of thyroglobin and larger iodoproteins in single rat thyroid follicles. Pflugers Arch 1977;372:145-8.
117. Kim PS, Bole D, Arvan P. Transient aggregation of nascent thyroglobulin in the endoplasmic reticulum: relationship to the molecular chaperone, BiP. J Cell Biol 1992;118:541-9.
118. Christis C, Fullaondo A, Schildknegt D, Mkrtchian S, Heck AJ, et al. Regulated increase in folding capacity prevents unfolded protein stress in the ER. J Cell Sci 2010;123:787-94.
119. Adkison LR, Taylor S, Beamer WG. Mutant gene-induced disorders of structure, function and thyroglobulin synthesis in congenital goitre (cog/cog) in mice. J Endocrinol 1990;126:51-8.
120. Kim PS, Kwon OY, Arvan P. An endoplasmic reticulum storage disease causing congenital goiter with hypothyroidism. J Cell Biol 1996;133:517-27.
121. Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature 1988;332:462-4.
122. Shiu RP, Pouyssegur J, Pastan I. Glucose depletion accounts for the induction of two transformation-sensitive membrane proteinsin Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A 1977;74:3840-4.
123. Lee AS. The accumulation of three specific proteins related to glucose-regulated proteins in a temperature-sensitive hamster mutant cell line K12. J Cell Physiol 1981;106:119-25.
125. Ordookhani A, Mirmiran P, Walfish PG, Azizi F. Transient neonatal hypothyroidism is associated with elevated serum anti-thyroglobulin antibody levels in newborns and their mothers. J Pediatr 2007;150:315-7. 7e2
126. Cho EM, Kim UH, Choi BH, Ko CW. Changes of antithroglobulin antibody in children with congenital hypothyroidism. Ann Pediatr Endocrinol Metab 2013;18:179-82.
127. Peteiro-Gonzalez D, Lee J, Rodriguez-Fontan J, Castro-Piedras I, Cameselle-Teijeiro J, et al. New insights into thyroglobulin pathophysiology revealed by the study of a family with congenital goiter. J Clin Endocrinol Metab 2010;95:3522-6.
128. Nicholas AK, Serra EG, Cangul H, Alyaarubi S, Ullah I, et al. Comprehensive screening of eight known causative genes in congenital hypothyroidism with gland-in-situ. J Clin Endocrinol Metab 2016;101:4521-31.
129. Citterio CE, Siffo S, Moya CM, Pio MG, Molina MF, et al. p.L571P in the linker domain of rat thyroglobulin causes intracellular retention. Mol Cell Endocrinol 2020;505:110719.
130. Citterio CE, Targovnik HM, Arvan P. The role of thyroglobulin in thyroid hormonogenesis. Nat Rev Endocrinol 2019;15:323-38.
131. Bernard C. Leçons sur les phénomenes de la vie communs aux animaux et aux végétaux. Paris: JB Bailliere et fils; 1878.
133. Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science 2008;319:916-9.
134. Kotas ME, Medzhitov R. Homeostasis, inflammation, and disease susceptibility. Cell 2015;160:816-27.
136. Targovnik HM, Scheps KG, Rivolta CM. Defects in protein folding in congenital hypothyroidism. Mol Cell Endocrinol 2020;501:110638.
137. Clairmont CA, De Maio A, Hirschberg CB. Translocation of ATP into the lumen of rough endoplasmic reticulum-derived vesicles and its binding to luminal proteins including BiP (GRP 78) and GRP 94. J Biol Chem 1992;267:3983-90.
138. Peth A, Uchiki T, Goldberg AL. ATP-dependent steps in the binding of ubiquitin conjugates to the 26S proteasome that commit to degradation. Mol Cell 2010;40:671-81.
139. Tsvetkov P, Myers N, Eliav R, Adamovich Y, Hagai T, et al. NADH binds and stabilizes the 26S proteasomes independent of ATP. J Biol Chem 2014;289:11272-81.
141. Patel A, Malinovska L, Saha S, Wang J, Alberti S, et al. ATP as a biological hydrotrope. Science 2017;356:753-6.
142. Zhuang ZX, Chen SE, Chen CF, Lin EC, Huang SY. Genome-wide association study on the body temperature changes of a broiler-type strain Taiwan country chickens under acute heat stress. J Therm Biol 2019;82:33-42.
143. Grases G, Pérez-Castelló JA, Sanchis P, Casero A, Perelló J, et al. Anxiety and stress among science students. Study of calcium and magnesium alterations. Magnes Res 2006;19:102-6.
144. Boreková M, Hojerová J, Koprda V, Bauerová K. Nourishing and health benefits of coenzyme Q10. Czech J Food Sci 2008;26:229-41.
145. Hsu JM, Root AW, Duckett GE, Smith JC Jr, Yunice AA, et al. The effect of magnesium depletion on thyroid function in rats. J Nutr 1984;114:1510-7.
146. Xu J, Tang S, Song E, Yin B, Wu D, et al. Hsp70 expression induced by Co-Enzyme Q10 protected chicken myocardial cells from damage and apoptosis under in vitro heat stress. Poult Sci 2017;96:1426-37.
147. Xu J, Yin B, Huang B, Tang S, Zhang X, et al. Co-enzyme Q10 protects chicken hearts from in vivo heat stress via inducing HSF1 binding activity and Hsp70 expression. Poult Sci 2019;98:1002-11.
148. Xu J, Tang S, Yin B, Sun J, Song E, et al. Co-enzyme Q10 and acetyl salicylic acid enhance Hsp70 expression in primary chicken myocardial cells to protect the cells during heat stress. Mol Cell Biochem 2017;435:73-86.
149. Nagashima Y, Iwata Y, Ashida M, Mishiba K, Koizumi N. Exogenous salicylic acid activates two signaling arms of the unfolded protein response in Arabidopsis. Plant Cell Physiol 2014;55:1772-8.
150. Poór P, Czékus Z, Tari I, Ördög A. The multifaceted roles of plant hormone salicylic acid in endoplasmic reticulum stress and unfolded protein response. Int J Mol Sci 2019;20.
151. Poór P. Effects of salicylic acid on the metabolism of mitochondrial reactive oxygen species in plants. Biomolecules 2020;10.
152. Gerschman R, Gilbert DL, Nye SW, Dwyer P, Fenn WO. Oxygen poisoning and x-irradiation: a mechanism in common. Science 1954;119:623-6.
153. Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol 1956;11:298-300.
154. Adam-Vizi V, Chinopoulos C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol Sci 2006;27:639-45.
155. Kalén A, Appelkvist EL, Dallner G. Age-related changes in the lipid compositions of rat and human tissues. Lipids 1989;24:579-84.
156. Green J, Edwin EE, Diplock AT, Bunyan J. Role of selenium in relation to ubiquinone in the rat. Nature 1961;189:748-9.
157. Hidiroglou M, Jenkins K, Carson RB, Brossard GA. Selenium and coenzyme Q10 levels in the tissues of dystrophic and healthy calves. Can J Physiol Pharmacol 1967;45:568-9.
158. Vadhanavikit S, Ganther HE. Decreased ubiquinone levels in tissues of rats deficient in selenium. Biochem Biophys Res Commun 1993;190:921-6.
159. Vadhanavikit S, Ganther HE. Selenium deficiency and decreased coenzyme Q levels. Mol Aspects Med 1994;15 Suppl:s103-7.
160. Xing L, Chen C, Li B, Yu H, Chai ZF. Distribution and location of selenium and other elements in different mitochondrial compartments of human liver by neutron activation analysis. J Radioanal Nucl Chem 2006;269:527-34.
161. Lee JH, Park KJ, Jang JK, Jeon YH, Ko KY, et al. Selenoprotein S-dependent Selenoprotein K binding to p97(VCP) protein is essential for endoplasmic reticulum-associated degradation. J Biol Chem 2015;290:29941-52.
162. Ren B, Liu M, Ni J, Tian J. Role of selenoprotein F in protein folding and secretion: potential involvement in human disease. Nutrients 2018;10.
163. Labunskyy VM, Yoo MH, Hatfield DL, Gladyshev VN. Sep15, a thioredoxin-like selenoprotein, is involved in the unfolded protein response and differentially regulated by adaptive and acute ER stresses. Biochemistry 2009;48:8458-65.
164. Hamieh A, Cartier D, Abid H, Calas A, Burel C, et al. Selenoprotein T is a novel OST subunit that regulates UPR signaling and hormone secretion. EMBO Rep 2017;18:1935-46.
165. Addinsall AB, Wright CR, Andrikopoulos S, van der Poel C, Stupka N. Emerging roles of endoplasmic reticulum-resident selenoproteins in the regulation of cellular stress responses and the implications for metabolic disease. Biochem J 2018;475:1037-57.
166. Ha HY, Alfulaij N, Berry MJ, Seale LA. From selenium absorption to selenoprotein degradation. Biol Trace Elem Res 2019;192:26-37.
167. Pitts MW, Hoffmann PR. Endoplasmic reticulum-resident selenoproteins as regulators of calcium signaling and homeostasis. Cell Calcium 2018;70:76-86.
168. Rocca C, Pasqua T, Boukhzar L, Anouar Y, Angelone T. Progress in the emerging role of selenoproteins in cardiovascular disease: focus on endoplasmic reticulum-resident selenoproteins. Cell Mol Life Sci 2019;76:3969-85.
169. Anouar Y, Lihrmann I, Falluel-Morel A, Boukhzar L. Selenoprotein T is a key player in ER proteostasis, endocrine homeostasis and neuroprotection. Free Radic Biol Med 2018;127:145-52.
170. Lange H, Kaut A, Kispal G, Lill R. A mitochondrial ferredoxin is essential for biogenesis of cellular iron-sulfur proteins. Proc Natl Acad Sci U S A 2000;97:1050-5.
171. Sipos K, Lange H, Fekete Z, Ullmann P, Lill R, et al. Maturation of cytosolic iron-sulfur proteins requires glutathione. J Biol Chem 2002;277:26944-9.
172. Cai K, Frederick RO, Kim JH, Reinen NM, Tonelli M, et al. Human mitochondrial chaperone (mtHSP70) and cysteine desulfurase (NFS1) bind preferentially to the disordered conformation, whereas co-chaperone (HSC20) binds to the structured conformation of the iron-sulfur cluster scaffold protein (ISCU). J Biol Chem 2013;288:28755-70.
173. Fuss JO, Tsai CL, Ishida JP, Tainer JA. Emerging critical roles of Fe-S clusters in DNA replication and repair. Biochim Biophys Acta 2015;1853:1253-71.
174. Ayaub EA, Tandon K, Padwal M, Imani J, Patel H, et al. IL-6 mediates ER expansion during hyperpolarization of alternatively activated macrophages. Immunol Cell Biol 2019;97:203-17.
175. Stehling O, Paul VD, Bergmann J, Basu S, Lill R. Biochemical analyses of human iron-sulfur protein biogenesis and of related diseases. Methods Enzymol 2018;599:227-63.
176. Vigani G, Pii Y, Celletti S, Maver M, Mimmo T, et al. Mitochondria dysfunctions under Fe and S deficiency: is citric acid involved in the regulation of adaptive responses? Plant Physiol Biochem 2018;126:86-96.
177. Burschel S, Kreuzer Decovic D, Nuber F, Stiller M, Hofmann M, et al. Iron-sulfur cluster carrier proteins involved in the assembly of Escherichia coli NADH:ubiquinone oxidoreductase (complex I). Mol Microbiol 2019;111:31-45.
178. Tsaousis AD. On the origin of iron/sulfur cluster biosynthesis in eukaryotes. Front Microbiol 2019;10:2478.
179. Desbats MA, Vetro A, Limongelli I, Lunardi G, Casarin A, et al. Primary coenzyme Q10 deficiency presenting as fatal neonatal multiorgan failure. Eur J Hum Genet 2015;23:1254-8.
180. O’Connor J, Bensky D. Acupuncture. A comprehensive text. Shanghai College of Traditional Medicine. Seattle: Eastland Press; 1982.
181. Porkert M, Ullmann C. Chinese Medicine. New York: Henry Holt; 1982.
182. Kaptchuk TJ. The web that has no weaver: understanding chinese medicine. New York: Congdon & Weed; 1983.
183. Matsumoto K, Birch S. Extraordinary vessels. Brookline: Paradigm Publications; 1986.
184. Ellis A, Wiseman N, Boss K. Grasping the wind. Brookline: Paradigm Publications; 1989.
185. Flaws B. Sticking to the point. Boulder: Blue Poppy Press; 1990.
186. Ross J. Acupuncture point combinations: The Key to Clinical Success: Churchill Livingstone; 1995.
187. Kirschbaum B. Die 8 außerordentlichen Gefäße in der traditionellen chinesischen Medizin. Uelzen: Medizinisch Literarische Verlagsgesellschaft mbH; 2000.
188. Deadman P, Al-Khafaji M, Baker K. A manual of acupuncture. Hove: Journal of Chinese Medicine Publications; 2001.
189. Hicks A, Hicks J, Mole P. Five element constitutional acupuncture. Edinburgh: Churchill Livingstone; 2004.
190. Maciocia G. The foundations of chinese medicine. a comprehensive text for acupuncturists and herbalists. Oxford: Elsevier; 2005.
191. Maciocia G. The channels of acupuncture: clinical use of the secondary channels and eight extraordinary vessels. Churchill Livinstone; 2006.
192. Smith RL, Soeters MR, Wust RCI, Houtkooper RH. Metabolic Flexibility as an adaptation to energy resources and requirements in health and disease. Endocr Rev 2018;39:489-517.
193. Morishita Y, Kabil O, Young KZ, Kellogg AP, Chang A, et al. Thyrocyte cell survival and adaptation to chronic endoplasmic reticulum stress due to misfolded thyroglobulin. J Biol Chem 2020; doi: 10.1074/jbc.RA120.012656.
194. Moncayo R, Moncayo H. Exploring the aspect of psychosomatics in hypothyroidism: the WOMED model of body-mind interactions based on musculoskeletal changes, psychological stressors, and low levels of magnesium. Woman Psychosomatic Gynaecol Obstet 2014;1:1-11.
195. Santos LR, Durães C, Ziros PG, Pestana A, Esteves C, et al. Interaction of genetic variations in NFE2L2 and SELENOS modulates the risk of Hashimoto’s thyroiditis. Thyroid 2019;29:1302-15.
196. Addinsall AB, Martin SD, Collier F, Conlan XA, Foletta VC, et al. Differential regulation of cellular stress responses by the endoplasmic reticulum-resident Selenoprotein S (Seps1) in proliferating myoblasts versus myotubes. Physiol Rep 2018;6:e13926.
197. Marañon G. La sangre en los estados tiroideos. Universidad Central (Madrid); 1911.
198. Marañon G. Le facteur émotionnel dans la pathogénie des états hyperthyroïdiens. Ann de Med 1921;60:81-93.
200. Fierro-Benítez R, Penafiel W, De Groot LJ, Ramirez I. Endemic goiter and endemic cretinism in the Andean region. N Engl J Med 1969;280:296-302.
201. Kim PS, Dunn AD, Dunn JT. Altered immunoreactivity of thyroglobulin in thyroid disease. J Clin Endocrinol Metab 1988;67:161-8.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Moncayo R, Moncayo H, Reisenzahn J. Global view on the pathogenesis of benign thyroid disease based on historical, experimental, biochemical and genetic data, identifying the role of magnesium, selenium, coenzyme Q10 and iron in the context of the unfolded protein response and protein quali. J Transl Genet Genom 2020;4:356-83. http://dx.doi.org/10.20517/jtgg.2020.32
AMA Style
Moncayo R, Moncayo H, Reisenzahn J. Global view on the pathogenesis of benign thyroid disease based on historical, experimental, biochemical and genetic data, identifying the role of magnesium, selenium, coenzyme Q10 and iron in the context of the unfolded protein response and protein quali. Journal of Translational Genetics and Genomics. 2020; 4(4): 356-83. http://dx.doi.org/10.20517/jtgg.2020.32
Chicago/Turabian Style
Moncayo, Roy, Helga Moncayo, Juliana Reisenzahn. 2020. "Global view on the pathogenesis of benign thyroid disease based on historical, experimental, biochemical and genetic data, identifying the role of magnesium, selenium, coenzyme Q10 and iron in the context of the unfolded protein response and protein quali" Journal of Translational Genetics and Genomics. 4, no.4: 356-83. http://dx.doi.org/10.20517/jtgg.2020.32
ACS Style
Moncayo, R.; Moncayo H.; Reisenzahn J. Global view on the pathogenesis of benign thyroid disease based on historical, experimental, biochemical and genetic data, identifying the role of magnesium, selenium, coenzyme Q10 and iron in the context of the unfolded protein response and protein quali. J. Transl. Genet. Genom. 2020, 4, 356-83. http://dx.doi.org/10.20517/jtgg.2020.32
About This Article
Copyright
Data & Comments
Data
 Cite This Article 14 clicks
Cite This Article 14 clicks




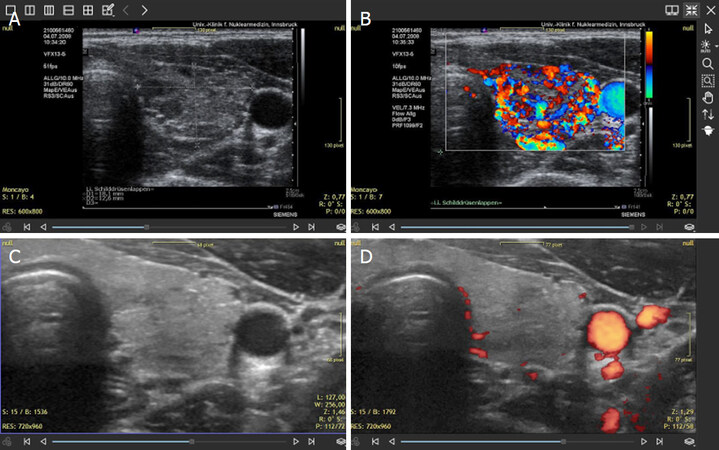
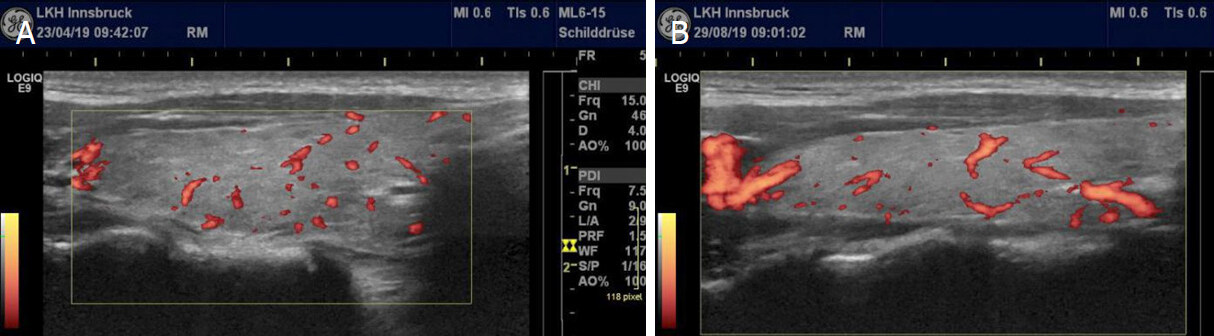
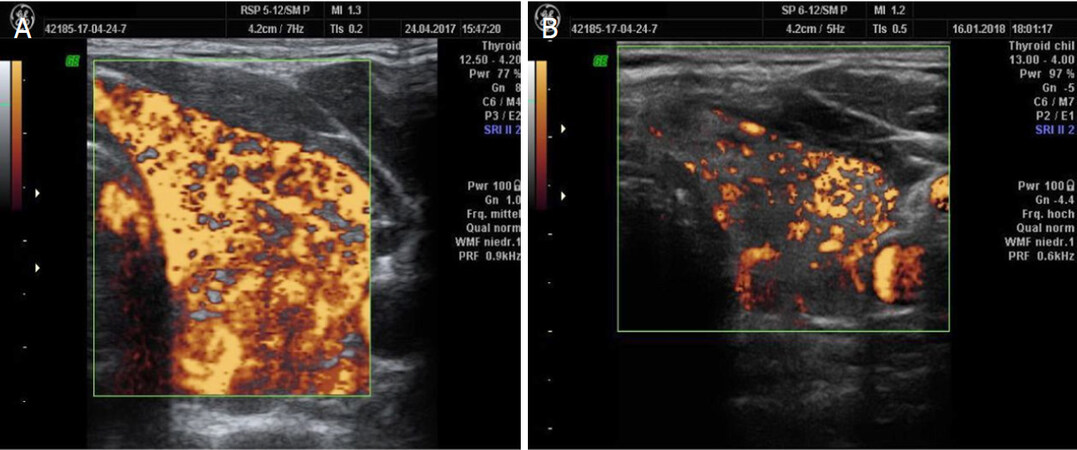
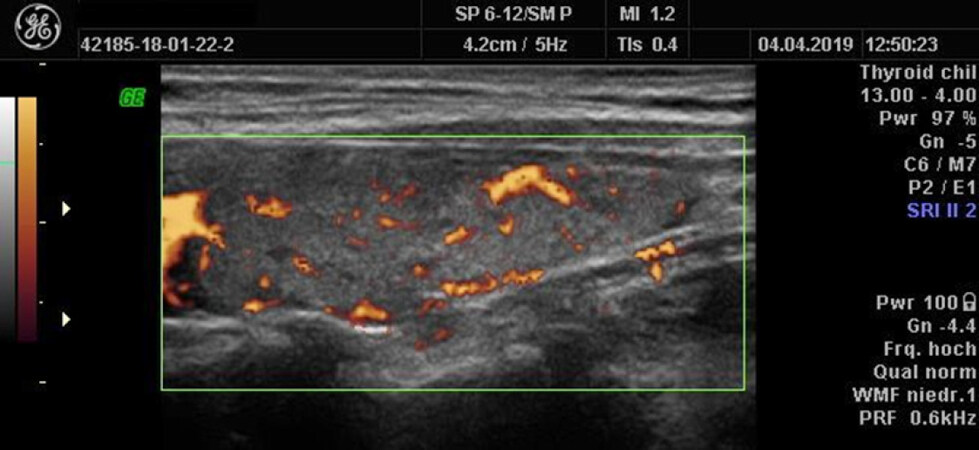
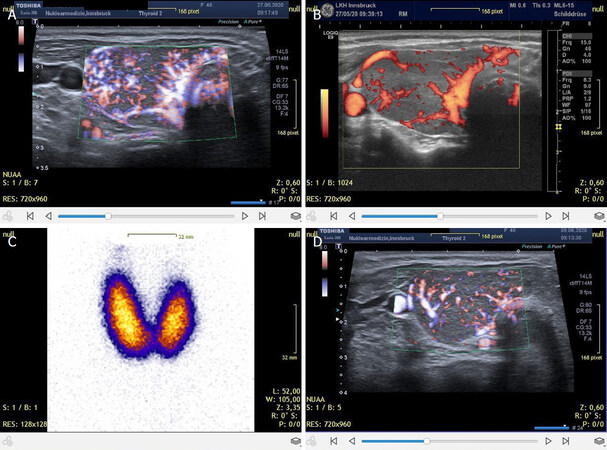
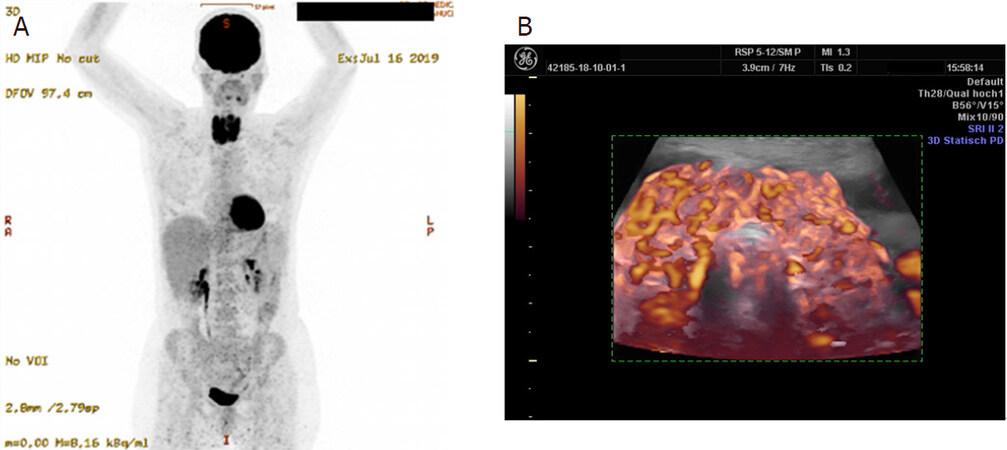
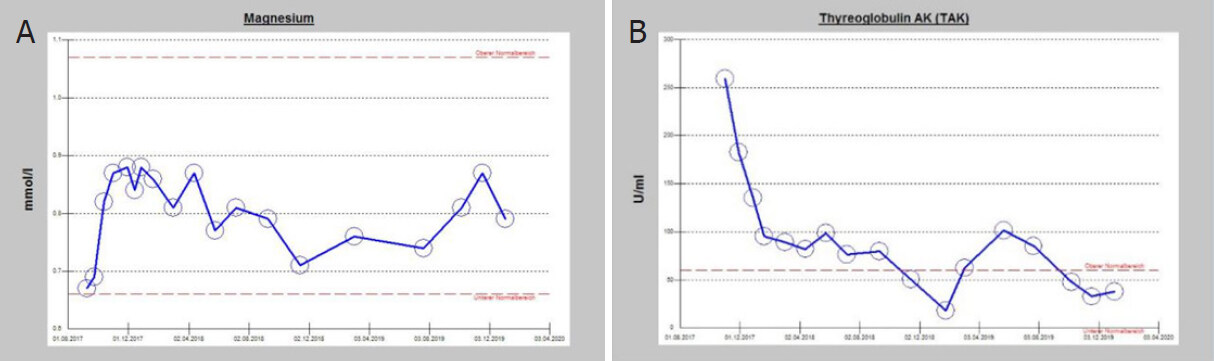
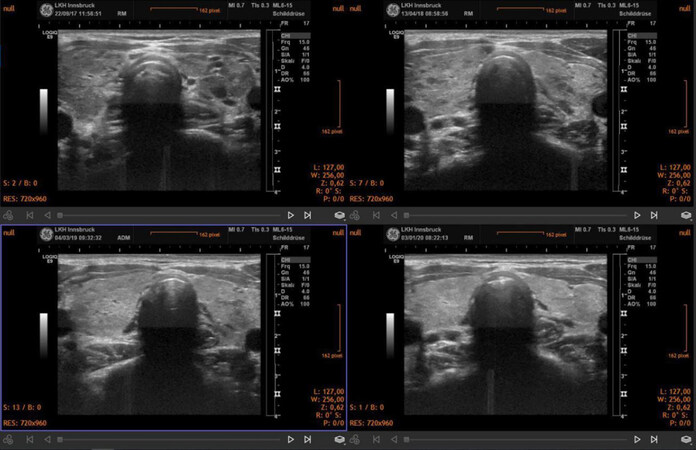
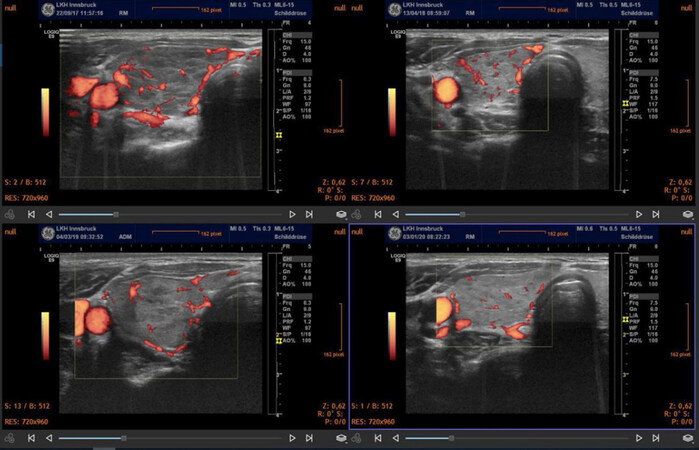
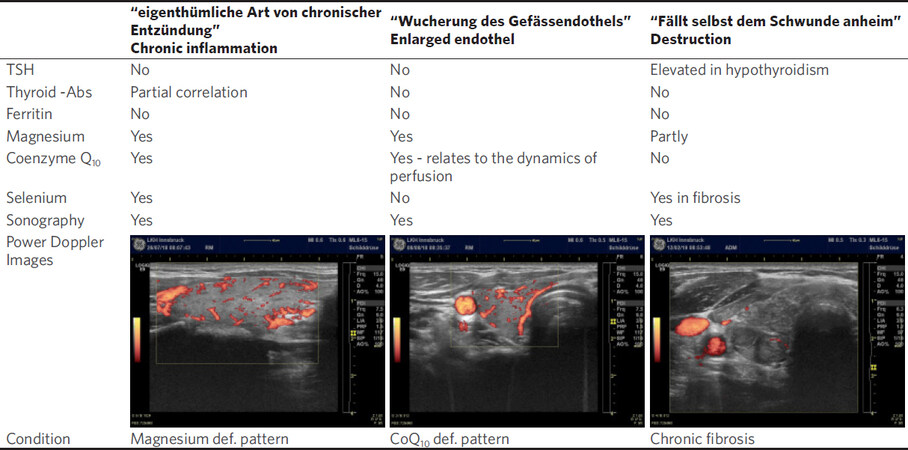
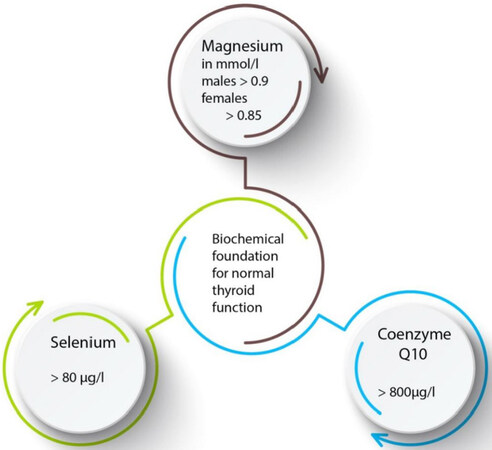
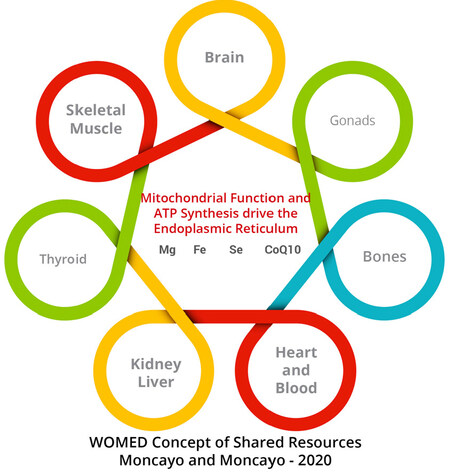
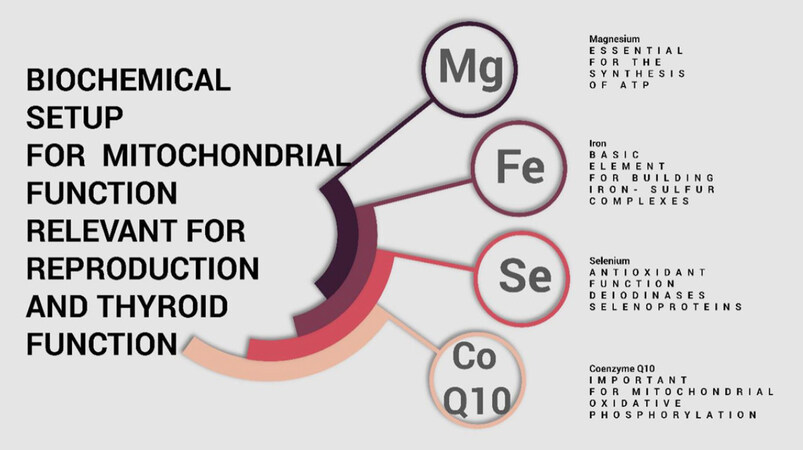









Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.