The endocrine and epigenetic impact of persistent cow milk consumption on prostate carcinogenesis
Abstract
This review analyzes the potential impact of milk-induced signal transduction on the pathogenesis of prostate cancer (PCa). Articles in PubMed until November 2021 reporting on milk intake and PCa were reviewed. Epidemiological studies identified commercial cow milk consumption as a potential risk factor of PCa. The potential impact of cow milk consumption on the pathogenesis of PCa may already begin during fetal and pubertal prostate growth, critical windows with increased vulnerability. Milk is a promotor of growth and anabolism via activating insulin-like growth factor-1 (IGF-1)/phosphatidylinositol-3 kinase (PI3K)/AKT/mechanistic target of rapamycin complex 1 (mTORC1) signaling. Estrogens, major steroid hormone components of commercial milk of persistently pregnant dairy cows, activate IGF-1 and mTORC1. Milk-derived signaling synergizes with common driver mutations of the PI3K/AKT/mTORC1 signaling pathway that intersect with androgen receptor, MFG-E8, MAPK, RUNX2, MDM4, TP53, and WNT signaling, respectively. Potential exogenously induced drivers of PCa are milk-induced elevations of growth hormone, IGF-1, MFG-E8, estrogens, phytanic acid, and aflatoxins, as well as milk exosome-derived oncogenic microRNAs including miR-148a, miR-21, and miR-29b. Commercial cow milk intake, especially the consumption of pasteurized milk, which represents the closest replica of native milk, activates PI3K-AKT-mTORC1 signaling via cow milk’s endocrine and epigenetic modes of action. Vulnerable periods for adverse nutrigenomic impacts on prostate health appear to be the fetal and pubertal growth periods, potentially priming the initiation of PCa. Cow milk-mediated overactivation of PI3K-AKT-mTORC1 signaling synergizes with the most common genetic deviations in PCa, promoting PCa initiation, progression, and early recurrence.
Keywords
INTRODUCTION
Prostate cancer (PCa) is the sixth leading cause of cancer death among men worldwide and is expected to reach 2.3 million new cases and 740,000 deaths by 2040[1]. The highest incidence rates are observed in Australia, New Zealand, North America, Western and Northern Europe, and the Caribbean. The lowest rates are found in South-Central Asia, Northern Africa, and Southeast and Eastern Asia[2,3]. According to the Global Cancer Statistics 2020 (GLOBOCAN), PCa contributes to 7.3% of all cancers[3]. The median age of PCa diagnosis is 68 years, while 10% of new cases in the USA are diagnosed in men aged ≤ 55 years[4]. Western diet and lifestyle have been linked to PCa prevalence[5-10]. Substantial lifestyle changes took place in Japan after World War 2, where a 20-fold increase in milk intake was associated with a 25-fold increased death rate of PCa[11].
Accumulated evidence confirms that cow milk consumption is positively related to the risk of PCa[12-14]. Milk and dairy products are substantial components of nutrition in Western industrialized countries as compared to Asian or North African countries [Figure 1][15]. In 2019, the per capita cow milk consumption in Germany was 49.5 L[16]. Higher milk consumption is reported in Scandinavian countries. In Sweden, the annual per capita milk consumption declined from 2007 to 2018 from 130.5 to 98.2 L[17]. The annual per capita milk consumption in the USA declined from 89.4 kg in 2000 to 64.0 kg in 2019[18]. In Asian populations, milk consumption is much lower. In 2019, Chinese people consumed on average only 12.5 kg of milk and dairy products per year[19].
Figure 1. (A) Per capita consumption (kg) of milk and milk-derived products worldwide in 2017. (B) Comparison of per capita consumption (kg) of milk and milk-derived products during 1961-2017 in Western Europe, the United States, and China according to Our World in Data[15].
It is the intention of this review to relate milk-derived signaling pathways with the molecular pathology of PCa. To understand milk’s impact on the pathogenesis of PCa, it is of key importance to appreciate milk’s biological nature as an endocrine and epigenetic system generated by the lactation genome to promote mechanistic target of rapamycin complex 1 (mTORC1)-driven postnatal growth, translation, and anabolism[20], a critical mode of cell signaling maintained by the consumption of commercial dairy milk[21]. Evidence is presented that the nutrigenomic signaling of milk converges with common oncogenic aberrations of PCa cells.
GENETIC DEVIATIONS ACTIVATING PI3K-AKT-mTORC1 IN PCa
Oncogenic activation of the phosphatidylinositol-3-kinase (PI3K)-AKT (protein kinase B)-mammalian target of rapamycin complex 1 (mTORC1) pathway is a frequent finding in PCa that promotes tumorigenesis, tumor progression, and resistance to therapy[22,23]. PI3K-AKT-mTORC1 signaling is elevated in a high proportion of PCa and castration-resistant PCa (CRPC)[24-27]. Reduced expression of phosphatase and tensin homolog deleted on chromosome 10 (PTEN), a critical tumor suppressor of PCa, correlates with PCa progression and poor prognosis[28]. In fact, PTEN is one of the most frequently deleted genes in PCa[29-34]. Notably, PTEN loss in primary PCa specimens correlates with high Gleason score and advanced disease[35]. Aberrant gene expression of PI3K pathway components is common in PCa and occurs in 42% and 100% of primary and metastatic PCa specimens, respectively[36-40]. It is of crucial importance that the PI3K-AKT-mTORC1 cascade interacts with androgen receptor (AR), RAS/mitogen-activated protein kinase (MAPK), and Wingless (WNT) signaling pathways[22]. Of note, AKT-mediated phosphorylation and nuclear extrusion of FoxO1, a nuclear suppressor of AR[41-45], activates androgen signaling[43,44]. Notably, AR regulates L-type amino acid transporters (LATs) that are of pivotal importance for the cellular uptake of mTORC1-activating branched-chain amino acids (BCAAs), especially leucine[46]. In comparison to other protein sources, whey proteins exhibit the highest amounts of leucine[47,48]. LAT1 and LAT3 mediate the uptake of leucine and other essential amino acids. PCa cells express LAT1 and LAT3 to maintain sufficient levels of leucine required for mTORC1-dependent cancer cell growth[46,49]. LAT inhibition decreased PCa cell growth and mTORC1 activity. AR-mediated LAT3 expression maintained levels of amino acid influx through ATF4 regulation of LAT1 expression after amino acid deprivation[46]. High levels of LAT3 are observed in primary disease, whereas increased levels of LAT1 are detected in PCa metastasis after hormone ablation[46]. Epidermal growth factor (EGF)-activated PI3K/AKT signaling also stimulates cellular leucine import through LAT3 in PCa cell lines[50]. LAT inhibition could thus be an effective therapeutic strategy against PCa[51,52].
Furthermore, overactivation of PI3K-AKT signaling attenuates the inhibitory effect of FoxO1 on RUNT-related transcription factor 2 (RUNX2) transcriptional activity, promoting PCa cell migration and invasion[53]. Increased RUNX2 activation has been related to metastatic disease[54], especially tumor progression in the bone[55,56].
The frequency of common genetic deviations of genes in the PI3K-AKT-mTORC1 pathway in PCa is presented in Table 1 according to the works of Shorning et al.[22] and Armenia et al.[39].
Frequency of common genetic alterations in the PI3K-AKT-mTORC1 pathway in PCa
| Altered genes | Frequency in PCa (%) |
| PTEN deletion/mutation | 16.4-32.0 |
| DEPTOR amplification | 5.1-21.4 |
| SGK mutation/amplification | 5.6-20.5 (SGK3) 0.2-2.7 (SGK1) |
| FOXO deletion | 0-15.2 (FOXO1) 4.5-13.4 (FOXO3) |
| MAP3K7 deletion | 5.9-14.8 |
| RRAGD deletion | 6.5-14.4 |
| SESN1 mutation/deletion | 5.4-13.6 |
| PIK3CA mutation/amplification | 5.5-11.5 |
| PIK3C2B mutation/amplification | 1.4-11.5 |
| PDPK1 amplification | 0-8.1 |
Overactivated mTORC1 signaling plays a crucial role in PCa initiation and progression[57-62].
Taken together, substantial evidence underlines the importance of genetic deviations in PCa cells and PCa cancer stem cells enhancing PI3K-AKT-mTORC1 signaling.
Epigenetic upregulation of PI3K-AKT-mTORC1 in PCa
Not only genetic but also epigenetic deviations contribute to PCa tumorigenesis and disease progression. Epigenetic alterations change DNA methylation, histone modifications, and the pattern of microRNA (miR) expression[63-68].
MicroRNA-21 in PCa
miR-21 is regarded as a critical oncomiR contributing to PCa carcinogenesis and progression[69,70]. miR-21 targets the mRNAs of key tumor suppressor genes including PTEN[71], programmed cell death 4 (PDCD4)[72], and forkhead box O1A[73,74]. Of note, FoxO3a, which is deregulated in PCa[75], inhibits the expression of miR-21[76]. In contrast, androgens enhance the expression of miR-21, and AR-regulated miR-21 promotes hormone-dependent and -independent PCa growth[77]. miR-21 and miR-375 of urinary exosomes have recently been reported to serve as biomarkers for the detection and prognosis of PCa[78,79]. In accordance, urine miR-21-5p is regarded as a potential non-invasive biomarker in patients with PCa[80,81]. Thus, upregulated miR-21 and miR-30c in serum/plasma emerged as biomarkers of PCa[82], as well as increased expression of miR-21 in peripheral blood mononuclear cells[83]. Furthermore, miR-21 is significantly upregulated in PCa compared to benign prostatic hyperplasia[84,85]. The expression of miR-21 and WNT-11 are associated with high Gleason scores in PCa tissues, promoting epithelial-mesenchymal transition (EMT) in aggressive PCa cells[86]. Further target genes of miR-21 are presented in Table 2.
Target genes of miR-21 related to the pathogenesis and progression of PCa
| miR-21 targets | Regulatory proteins | Ref. |
| PTEN | Phosphatase and tensin homolog | [71] |
| PDCD4 | Programmed cell death 4 | [72] |
| FOXO1A | Forkhead box transcription factor O1a | [73,74] |
| KLF5 | Kruppel-like factor 5 | [87] |
| IGFBP3 | IGF binding protein 3 | [88] |
| CDKN1C | Cyclin-dependent kinase inhibitor 1C | [89] |
| MARCKS | Myristoylated alanine-rich protein kinase C substrate | [90] |
| TGFBR2 | Transforming growth factor β-receptor II | [91] |
| FBXO11 | F-box only protein 11 | [92] |
| RECK | Reversion-inducing cysteine-rich protein with KAZAL motifs | [93] |
Thus, there is compelling evidence that miR-21 including circulating miR-21, which is elevated in the serum and exosomes of PCa patients, plays an important role in PCa carcinogenesis, progression, and metastasis[75-95].
MicroRNA-148a in PCa
miR-148a is another oncogenic miR that plays a pivotal role in PCa[96-99]. Increased levels of miR-148a-3p in serum are associated with PCa[96]. miR-148a-3p expression is increased in PCa tissue and exhibits higher expression levels correlating with increased Gleason score[97]. miR-148a is an AR-responsive miR promoting LNCaP prostate cell growth by repressing its target cullin-associated neddylation-dissociated protein 1[99]. A significant growth advantage for LNCaP cells transfected with pre-miR-148a was found with significantly increased numbers of cells in the S phase[100]. miR-148a silences cyclin-dependent kinase inhibitor 1B. miR-148a transfection into LNCaP cells increased S-phase transition and enhanced cell proliferation[101]. Further target genes of miR-148a are B-cell translocation gene 2 and phosphatidylinositol 3-kinase-interacting protein 1 (PIK3IP1)[100]. PIK3IP1 directly binds to the p110 catalytic subunit of PI3K and downregulates PI3K activity[102]. PIK3IP1 negatively regulates PI3K activity and thereby suppresses activation of AKT[102]. miR-148a-mediated suppression of PIK3IP1 thus enhances PI3K-AKT-mTORC1 signaling. It has recently been demonstrated that the E2F transcription factor 1 (E2F1)/DNA methyltransferase 1 (DNMT1) inhibitory axis of AR transcription is activated during the emergence of CRPC[103]. It has been shown that miR-148a-mediated suppression of DNMT1 induces the expression of apoptotic genes in hormone-refractory PCa cells[104]. In contrast, Lee et al.[105] provided evidence that reduced expression of DNMT1 was associated with EMT induction and cancer stem cell phenotype, enhancing tumorigenesis and metastasis of PCa. In a synergistic fashion with miR-21, miR-148a suppresses the expression of PTEN and DNMT1[98,106-110]. miR-21- and miR-148a-mediated suppression of DNMT1 with consecutive promoter gene demethylation increases the expression of insulin (INS)[111], IGF-1 (IGF1)[112,113], and mechanistic target of rapamycin (TOR)[114]. These are developmental genes of the insulin/IGF-1/PI3K/AKT/mTORC1 signaling cascade, which is upregulated in PCa. Collectively, there is compelling evidence that both miR-21 and miR-148a modify epigenetic regulation of PCa, enhancing PI3K-AKT-mTORC1 signal transduction.
MILK-INDUCED PI3K-AKT-mTORC1 SIGNALING
Calcium
Earlier studies suspected dairy calcium as a promoter of PCa pathogenesis[115]. The milk calcium content differs between cattle breeds, exhibiting the lowest calcium content in Holstein-Friesian (1275.0 ±
Calcium-independent milk-induced mTORC1 activation
Current research interest focuses on the pathogenic role of milk-induced elevations of IGF-1 in PCa[125]. In fact, overwhelming evidence accumulated over two decades supports the view that increased circulating levels of IGF-1 as well as local IGF-1/IGF1 receptor (IGF1R) signaling promote PCa initiation and progression[126-151]. The perception of milk has changed from a “pure food source” to an “endocrine and epigenetically active biologic system”, promoting IGF-1-PI3K-AKT-mTORC1 signaling substantially augmented by milk’s exosomal miRs[20,152,153]. Milk signaling functionally synergizes with dominant oncogenic driver mutations of PCa including androgen signaling, disturbed DNA repair, and mutations enhancing PI3K/AKT/mTORC1 signaling[21,154].
Despite recent progress in milk’s molecular biology and its sophisticated physiological functions[20,152,153], nutrition science and dairy industry-supported reviews present a positive view on milk as a nutrient for metabolic health, providing valuable proteins, macronutrients, oligosaccharides, calcium, vitamins, and other micronutrients[155-159]. None of those studies in the field of nutrition and epidemiological research appreciates milk’s biological role as an mTORC1-driving system of mammalian evolution physiologically restricted to the postnatal growth period[20,152,153,160]. In fact, there is growing evidence in various disciplines of medicine that milk consumption is associated with adverse health effects, increasing overall mortality[161-165]. Milk consumption has been related to several common mTORC1-driven cancers of Western civilization, especially PCa[166-175], breast cancer[176-183], hepatocellular carcinoma[184-187], and diffuse large B-cell lymphoma[188]. Notably, overactivated mTORC1 signaling is a common hallmark of PCa[21,22,27,57-61], breast cancer[189-194], hepatocellular carcinoma[195-200], and diffuse large B-cell lymphoma[201-204]. Based on a recent review of the literature, Vasconcelos et al.[154] confirmed a possible relationship between milk consumption and mTORC1-mediated initiation and progression of PCa.
Oncogenic activation of the PI3K/AKT/mTORC1 pathway is a frequent aberration in PCa pathogenesis[22,23]. There is an intimate crosstalk between the PI3K/AKT/mTORC1 cascade and multiple other signaling pathways that promote PCa progression[22,23]. Specifically, PI3K/AKT/mTORC1 signaling cooperates with AR, MAPK, and WNT signaling cascades[22,23]. To elucidate milk’s impact on mTORC1-dependent translation[20] and PCa initiation and progression[21], deeper insights into milk’s signaling pathways are mandatory.
mTORC1 is the cell’s central hub for the regulation of nutrient- and growth factor-dependent cell growth and anabolism[205-211]. To fulfill its biological function as promotor of postnatal growth, milk activates five major pathways stimulating mTORC1 via: (1) growth factors including growth hormone (GH), insulin, and IGF-1; (2) amino acids, especially BCAAs; (3) milk fat-derived palmitic acid; (4) the milk sugar lactose β-D-galactopyranosyl-(1→4)-D-glucose; and (5) epigenetic modifiers, especially milk exosome (MEX)-derived miRs. Activated PI3K-AKT-mTORC1 signaling in PCa is presented in Figure 2A. Figure 2B illustrates superimposed milk and milk miR signaling over-activating the PI3K-AKT-mTORC1 signaling cascade in PCa cells.
Figure 2. (A) Synopsis of PI3K-AKT-mTORC1 signaling pathways activated in prostate cancer (PCa) cells. (B) Superimposed nutrigenomic effects of milk signaling. Milk-induced and milk-derived hormones (insulin, GH, IGF-1, MFG-E8, and estrogens) activate PI3K and AKT, which in turn activate mTORC1.
Milk-derived essential amino acids (prototype leucine) activate mTORC1, increasing cell proliferation. MEX-derived miRs augment mTORC1 signaling and modify transcriptional activity in PCa. miR-148a and miR-21 suppress PTEN, which is commonly mutated or deleted in PCa. Increased AKT activity results in nuclear translocation of FoxO1, a key nuclear suppressor of AR, RUNX2, and sterol regulatory element binding protein 1. Milk-derived estrogens (E) may induce further transcription of IGF-1. RUNX2 is upregulated in PCa and controls the transcription of prostate specific antigen (PSA) and promotes bone metastasis. Phytanic acid activates γ-secretase, which cleaves CD44 releasing CD44 intracellular domain (CD44-ICD) that functions as a nuclear transcription factor cooperating with RUNX2. MEX-derived
Growth hormone
Milk consumption enhances GH levels in children and peak GH levels in adults[212,213]. Already two decades ago, the GH-IGF-1 axis was implicated in prostate carcinogenesis[214]. PCa cells express both GH and GH receptor (GHR)[215-217]. As shown in LNCaP cells, GH induces time- and dose-dependent signaling events. These include phosphorylation of Janus kinase 2, GHR, and signal transducer and activator of transcription 5, p42/p44 MAPK and AKT, respectively. Furthermore, GH modifies AR expression[215]. In androgen-dependent LNCaP cells, estradiol (E2), cortisol, and IGF-1 and IGF-2 all stimulate GH binding[216]. Human GH promotes IGF and β-E2 receptor (ERβ) gene expressions and interacts with IGF-1 and E2 to stimulate androgen-dependent LNCaP cell proliferation[217]. Furthermore, exogenous and autocrine GH augments the migration and invasion of LNCaP cells, dependent upon PI3K, STAT5, and MEK1/2 pathways[218].
In contrast, patients with Laron syndrome, who present dwarfism due to a genetic loss-of-function mutation of GHR[219,220], exhibit failures in the GH-GHR-IGF-1 signal transduction process, resulting in congenital IGF-1 deficiency associated with a reduced incidence of cancers including PCa[221-224]. Notably, disruption of GH signaling retards early stages of prostate carcinogenesis in the C3(1)/T antigen mouse and Probasin/TAg rat model[225,226]. GH-releasing hormone receptor antagonists decreased cell viability and provoked a reduction in proliferation in LNCaP and PC3 cells[227]. Recent evidence indicates that GH induction following androgen deprivation therapy or AR inhibition may contribute to the CRPC progression by bypassing androgen growth requirements[228]. In fact, GH induces expression of the AR splice variant 7, which correlates with antiandrogen resistance and induces IGF-1 that is implicated in PCa progression and ligand-independent AR activation[228]. Increased GH signaling via milk consumption may thus promote PCa development and CRPC progression.
Insulin-like growth factor 1
Milk consumption increases circulating IGF-1 levels in children, adolescents, and adults[212,213,229-235]. Whereas a cross-sectional study in Bavaria of 526 men and women aged 18-80 years showed that only milk but not yogurt and cheese intake increased serum IGF-1 levels[234], a larger British cohort study including 11,815 participants reported that milk and yogurt protein, but not cheese protein, increased serum IGF-1 concentrations[235]. Hoppe et al.[236] observed an increase in serum insulin by isolated consumption of whey protein and an increase of IGF-1 by isolated casein supplementation after seven days in prepubertal boys.
IGF-1 is a component of human and bovine milk[237-239]. Bovine IGF-1 exhibits an identical amino acid sequence compared to human IGF-1[240]. The GH-IGF-1 axis is of physiological importance for infant growth[241,242]. Of note, it is not the oral uptake of bovine GH and IGF-1 in dairy milk that increases serum IGF-1 levels, but the induction of hepatic IGF-1 synthesis and release after milk consumption[212,239]. Tryptophan, a major amino acid enriched in milk proteins, is the precursor of serotonin [5-hydroxytryptamine (5-HT)], which via 5-HT2 receptors stimulates hypothalamic GHRH release and pituitary GH secretion, thus increasing serum GH levels[243]. Hepatic GH/GHR signaling is the major stimulus increasing circulatory IGF-1 levels [Figure 2][244,245].
The milk protein-derived amino acids tryptophan, methionine, and arginine synergistically enhance hepatic IGF-1 synthesis and secretion[212,246-252]. Exposure to bovine MEX to cultured human colonic LS174T cells enhanced the expression of glucose-regulated protein 94 (GRP94)[253], the most abundant intraluminal endoplasmic reticulum chaperone that aids in the synthesis of IGF-1, IGF-2, and proinsulin[254,255].
IGF-1 activates the PI3K-AKT pathway. Activated AKT phosphorylates tuberin (TSC2), which promotes the dissociation of TSC2 from the lysosomal membrane activating RAS homolog enriched in brain (RHEB). RHEB finally activates mTORC1 at the lysosomal membrane[207,210,256-260]. IGF-1 is a pivotal promoter of linear growth and body size[261-265].
Milk protein-derived insulinotropic BCAAs are released by intestinal hydrolysis. They induce postprandial hyperinsulinemia explaining milk’s high insulinemic index compared to its low glycemic index[266,267]. Fast intestinal hydrolysis of whey protein-derived amino acids contributes to milk’s high insulinemic effect[236,268-271]. In a synergistic fashion, insulin and IGF-1 activate PI3K-AKT-mTORC1 signaling and thereby promote growth and anabolism of target tissues[257,259,272-276].
Amino acids
Compared to other protein sources, milk protein and casein contain high amounts leucine and methionine. Compared to meat, whey proteins are highly enriched in leucine[47,277]. In addition, milk protein exhibits a high glutamine content (8.1 g/100 g protein) compared to beef (glutamine 4.75 g/100 g protein)[278]. Glutamine via the glutaminolysis pathway also results in mTORC1 activation[279,280]. Major milk-derived amino acids such as leucine, arginine, and methionine are sensed via sestrin 2, cellular arginine sensor for mTORC1, and S-adenosylmethionine sensor upstream of mTOR, respectively. These amino acids stimulate mTORC1 activation through RAG GTPase pathways[281-298]. Glutamine activates mTORC1 through a RAG GTPase-independent mechanism that requires ADP-ribosylation factor 1 (ARF1)[297]. Leucyl-tRNA synthetase (LRS) is another amino acid-dependent regulator of mTORC1[299,300]. LRS senses intracellular leucine concentration and directly binds to RAG GTPase, the mediator of amino acid signaling to mTORC1, in an amino acid-dependent manner and functions as a GTPase-activating protein for RAG GTPase to activate mTORC1[300]. Moreover, LRS operates as a leucine sensor for the activation of the class III PI3K Vps34 that mediates amino acid signaling to mTORC1 by regulating lysosomal translocation and activation of the phospholipase PLD1[301]. mTORC1 activation involves leucine sensing, LRS translocation to the lysosome, and interaction with RAGD[302-305]. There is a further function of LRS1 in glucose-dependent control of leucine usage[306]. Upon glucose starvation, LRS1 is phosphorylated by Unc-51-like autophagy activating kinase 1 at the residues crucial for leucine binding. Phosphorylated LRS1 decreases leucine binding, which may inhibit protein synthesis thereby saving energy[306].
In addition, arginine relieves allosteric inhibition of RHEB by TSC[307]. Arginine cooperates with growth factor signaling, which further dissociates TSC2 from lysosomes and activates of mTORC1[307].
Taken together, full mTORC1 activation only occurs when both RAG and RHEB GTPase pathways are fully activated, neither being sufficient alone[295]. The final activators of growth factor and amino acids signaling pathways, RHEB and RAGs, converge at the lysosome to activate mTORC1 [Figure 2][281-296,308].
Palmitic acid
The predominant saturated fatty acid of milk triacylglycerols (TAGs) is palmitic acid (C16:0), which is transported in milk fat globules (MFGs)[309,310]; MFGs, in turn, transfer energy through their TAG core[311]. After intestinal TAG hydrolysis and re-esterification into chylomicrons, palmitic acid serves as an energy source and fuels mitochondrial β-oxidation for ATP synthesis[312,313]. ATP inhibits AMP-activated protein kinase (AMPK) and thereby activates mTORC1[314-316]. As shown in skeletal muscle cells, palmitate activates mTORC1/p70S6K signaling by Raptor phosphorylation[317], stimulates mTORC1 activation at the lysosome[318,319], and induces lipid deposition in HepG2 cells via activation of the mTORC1/S6 kinase 1 (S6K1)/SREBP-1c pathway[320].
It has been shown by dynamic microfluidic Raman technology that palmitic acid and arachidonic acid exhibit a high uptake in PC3 cells, whereas docosahexaenoic acid and eicosapentaenoic acid have inhibitory effects on the uptake of palmitic acid and arachidonic acid[321]. The Japan Public Health Center-Based Prospective Study Group reported relative PCa risks [95% confidence intervals (CI)] on comparison of the highest with the lowest quartiles of myristic acid and palmitic acid of 1.62 (1.15-2.29) and 1.53 (1.07-2.20), respectively[322]. A recently published study in the United States including 49,472 men with an average follow-up period of 11.2 years and a median total dairy intake of 101 g/1000 kcal showed that regular fat dairy product intake was associated with late-stage PCa risk (HR = 1.37, 95%CI: 1.04-1.82 comparing the highest with lowest quartile) and 2% fat milk intake with advanced PCa risk (HR = 1.14, 95%CI: 1.02-1.28 comparing higher than median intake with no intake group)[323].
Milk fat globule-EGF factor 8
MFG membrane proteins, predominantly MFG epidermal growth factor 8 (MFG-E8, also known as lactadherin), stimulates cell proliferation through the PI3K/AKT/mTORC1 signaling pathway[324-327]. Increased levels of MFG-E8 found in tissue and plasma exosomes from PCa patients have been compared with controls[327,328]. Notably, M2 polarization of PCa-associated macrophages is induced by MFG-E8-mediated efferocytosis[327]. Of note, MFG-E8 is a major constituent of MFG membranes[329], but it is detectable only in minor amounts in milk EVs and exosomes[326-328]. However, MFG-E8 mRNA has been detected in bovine MEX[329-332]. Remarkably, MFG-E8 promotes the absorption of dietary TAGs and the cellular uptake of fatty acids and is linked to obesity[333]. MFG-E8 coordinates fatty acid uptake through αvβ3 integrin- and αvβ5 integrin-dependent phosphorylation of AKT by PI3K and mTOR complex 2, leading to translocation of CD36 antigen and fatty acid transport protein 1 from cytoplasmic vesicles to the cell surface[333]. Thus, MFG-E8 plays a key role in the absorption and storage of dietary fats[333]. Recent evidence indicates that insulin receptor signaling is autoregulated through MFG-E8 and the αvβ5 integrin[334]. Intriguingly, αvβ5 expression is upregulated in PCa and PCa cells[335,336], and the differentiation of PCa seems to influence integrin expression and subcellular distribution[335]. Notably, αvβ5 integrin expression is increased in plasma and urine EVs derived from PCa patients[337]. Whole milk, especially milk fat with enriched amounts of MFG-E8, may additionally promote PI3K/AKT/mTORC1 signaling in PCa [Figure 2].
Phytanic acid
The lipid fraction of milk contains phytanic acid (3,7,11,15-tetramethyl-hexadecanoic acid) representing 0.7% of milk total long-chain fatty acids[338]. Depending on the intensity of grass feeding, the content of phytanic acid varies from 9.7 mg/100 g in whole milk to 200 mg/100 g total milk lipids and represents a marker of organic milk production[339-341]. Higher phytanic acid intake, although unrelated to the risk of localized PCa, was associated with increased risks of advanced PCa[342]. It has been demonstrated in rat aortic smooth muscle cells that phytanic acid reduces the expression of IGF-1 receptor but increases γ-secretase activity[343]. Notably, γ-secretase mediates the intramembranous cleavage of CD44[344], a major adhesion molecule for the extracellular matrix components that is implicated in a wide variety of physiological and pathological processes including the regulation of EMT, cancer growth, and metastasis[345-351]. CD44 intracellular domain (CD44-ICD) acts as a signal transduction molecule translocating into the nucleus[352]. A strong functional relationship between CD44-ICD and RUNX2 has recently been shown in AR-positive PC3 cells[353]. In the nucleus, CD44-ICD and RUNX2 interact, and this interaction was higher in PC3 cells transfected with RUNX2 cDNA. In contrast, inhibition of CD44 cleavage with a γ-secretase inhibitor reduced the formation of CD44-ICD. Overexpression of RUNX2 augmented the expression of metastasis-related genes (e.g., MMP-9 and osteopontin), which resulted in increased migration and tumorsphere formation[353]. There is compelling evidence that RUNX2 enhances cell growth and responses to androgen and TGFβ in PCa cells[354]. Remarkably, RUNX2 stimulates AR responsive expression of the PSA[354]. Both RUNX1 and RUNX2 cooperate with prostate-derived ETS factor to activate the transcription of PSA upstream regulatory region[355]. Recently, a cooperation between AR and RUNX2 in the stimulation of oncogenes such as invasion-promoting SNAIL family transcription factor SNAI2 has been demonstrated[356]. RUNX2 not only is a master organizer of gene transcription in developing and maturing osteoblasts[357], which is related to the physiological function of milk increasing linear and skeletal growth[261,262], but it also promotes PCa bone metastasis[54,55,358]. It is thus of critical concern that cow milk-derived phytanic acid may induce γ-secretase-mediated CD44-ICD-RUNX2 nuclear signaling[56]. Of importance, Baier et al.[359] demonstrated that the expression of RUNX2 increased by 31% in blood mononuclear cells 6 h after commercial cow milk consumption in adult healthy volunteers compared to baseline.
Plasma phytanic acid concentrations have been significantly associated with intake of dairy fat[360]. Higher phytanic acid intake, although unrelated to the risk of localized PCa, was associated with increased risks of advanced PCa predominantly by phytanic acid obtained from dairy products[342], whereas no overall association has been detected between serum phytanic and pristanic acid levels and PCa risk[342,361]. In contrast, Xu et al.[362] reported that serum levels of phytanic acid among PCa patients were significantly higher than those of unaffected controls, suggestive of an association between phytanic acid and PCa risk [Figure 2].
Increasing evidence links PCa risk with polymorphisms in the α-methylacyl-CoA racemase (AMACR) gene and branched-chain fatty acids derived from specific sources of dietary fats[363-366]. AMACR is a catalyst in peroxisomal β-oxidation of branched-chain fatty acids found in milk and dairy products[367]. AMACR expression is actually downregulated in hormone-refractory metastatic tissue relative to the primary tumor[368]. An association between low AMACR expression at diagnosis and an increased risk of biochemical recurrence and fatal PCa has been reported[369]. Furthermore, lower AMACR intensity was associated with higher PSA levels and more advanced clinical stage at diagnosis, and there was a non-significant trend for higher risk of lethal outcomes[370]. In contrast, other studies report AMACR overexpression as an early event in prostate tumorigenesis that may precede morphologic evidence of malignant transformation[371,372].
Estrogens
Dairy cows continuously lactate throughout almost their entire pregnancy, explaining increased amounts of estrogens and progesterone in commercial milk[373]. A significant increase in serum estrone (E1) and progesterone levels has been shown in men and children who consumed 600 mL/m2 of cow milk. In addition, urine levels of E1, estradiol (E2), estriol (E3), and pregnanediol significantly increased in all consumers, allowing the conclusion that milk-derived estrogens were absorbed[373]. Milk of Swiss Holstein cows exhibited average hormone levels of E1 = 159 ng/kg, 17β-E2 = 6 ng/kg, 17α-E2 = 31 ng/kg, 4-androstenedione = 684 ng/kg, progesterone = 15,486 ng/kg, 17-hydroxyprogesterone = 214 ng/kg, cortisol = 235 ng/kg, and cortisone = 112 ng/kg, whereas E3 was below the limit of detection[374]. According to Malekinejad et al.[375], the major cow milk estrogens were free and deconjugated E1 (6.2-1266 ng/L), α-E2 (7.2-322 ng/L), and β-E2 (5.6-51 ng/L), whereas E3 was below the detection limit. The calculated daily estrogen intake through milk consumption was 372 ng[375]. In commercial milk samples, Tso et al.[376] confirmed that E1 (23-67 ng/L) was the major free estrogen, whereas E2 and E3 concentrations were below the limit of detection. Several conjugated estrogen metabolites were identified: 17β-E2-3-glucuronide (71-289 ng/L), E1-3-sulfate (60-240 ng/L), 17β-E2-3,17β-sulfate (< LOD to 30 ng/L), and E3-glucuronide (< LOD of 25 ng/L)[376]. Thus, endogenous and exogenous steroids derived from dairy products produced from whole milk are a source of exogenous steroid exposure to humans[377].
Obesity has been proposed to be involved in the pathogenesis and more aggressive courses of PCa[378,379]. Notably, a twofold elevation of serum E1 and 17β-E2 levels was observed in a group of morbidly obese men[380]. Thus, obese men with increased endogenous estrogen production may represent a vulnerable group, especially during further exogenous estrogen exposure derived from milk and dairy products. There is recent concern that estrogens represent an under-recognized contributor in PCa development and progression[381,382]. Of notice, AR and ERα expression changes during PCa progression, both independently and co-expressed[383]. In high-grade prostatic intraepithelial neoplasia specimens (epithelium and stroma), an increase in the population of double-positive (AR+/ERα+) cells has been observed, whereas double-negative (AR-/ERα-) cells significantly decreased in advanced PCa, from 65% in benign prostate tissue to 30% in metastasis tissue[383].
There is extensive crosstalk among estrogens, androgens, and IGF-1 signaling in PCa cells. In LNCaP and PC3 cells, E1 increased the level of AR and IGF-1 expression[384,385]. The upregulation of ERα and estrogen-regulated progesterone receptor (PR) during PCa progression and hormone-refractory PCa suggests that estrogens and progestins stimulate tumor growth[386]. A potentially aggressive molecular subtype of PCa exhibiting TMPRSS2-ERG gene fusion is regulated by ER. TMPRSS2-ERG expression increased by ERα agonist but decreased by ERβ agonists[386]. Thus, increasing evidence demonstrates that local estrogens contribute to prostate carcinogenesis and tumor progression[387]. It has also been reported in breast and renal carcinoma cells that estrogens and IGF-1 have synergistic effects on tumor cell growth[388-391]. In fact, IGF1R expression in PCa is upregulated by both androgens and estrogens sensitizing PCa cells to the mitogenic effects of IGF-1[392]. Of note, mTORC1 has been identified as a critical checkpoint for estrogen signaling[393]. The downstream effector of mTORC1, the ribosomal S6K1, activates ERα via phosphorylation of S167. ERα binding to Raptor promotes its translocation into the nucleus upon estrogen stimulation. In addition, phosphorylation of ERα on S104/106 by mTOR kinase activates transcription of ER target genes[394]. The molecular crosstalk between mTORC1 and ERα in the pathogenesis of PCa may be augmented by milk-mediated estrogens and milk-induced IGF-1, which synergistically activate mTORC1. Treatment of LNCaP cells with androgen or E2 triggers simultaneous association of AR and ERβ with SRC oncogene, activates the SRC/RAF-1/ERK-2 pathway, and stimulates cell proliferation[394]. It has been demonstrated in cortical neurons that ER protein interaction with p85, the regulatory unit of PI3K, leads to activation of AKT and ERK1/2[395]. PCa stem cells lack AR explaining the resistance to androgen deprivation therapy[396]. However, PCa stem cells express classical (α and/or β) and novel (GPR30) ERs[396]. Gene expression profiles of CD133+ 4/CD44+ PCa stem cells showed that many ribosomal proteins and translation initiation factors that constitute the mTOR complex were highly expressed[62].
Galactose
The lactose content of milk makes up around 2%-8% by weight. Lactose hydrolysis provides glucose and galactose, which both activate mTORC1. The great majority of men in Western societies consuming milk and dairy products is lactose-tolerant and can hydrolyze the disaccharide lactose into glucose and galactose. The total galactose content of bovine milk is 2.4 g/100 g[397]. Increased sugar consumption from sweetened beverages was associated with increased risk of PCa for men in the highest quartile of sugar consumption (HR = 1.21, 95%CI: 1.06-1.39), and there was a linear trend (P < 0.01)[398]. In adults, hepatic galactose elimination capacity is related to body weight and decreases slowly with age[399,400]. Notably, galactose increases oxidative stress, which has recently been linked to increased all-cause mortality by consumption of non-fermented milk[162-165]. Galactose is a mitochondrial stressor experimentally used for the induction of aging and neurodegeneration[401-404]. Disturbed oxidant/antioxidant balance has been implicated in the pathophysiology of PCa, especially in high-risk PCa subjects[405]. The ginsenoside Rg1 decreases oxidative stress and downregulates AKT/mTORC1 signaling and attenuates cognitive impairment in mice and senescence of neural stem cells induced by galactose[406]. Accumulated evidence underlines that oxidative stress is of critical importance in prostate carcinogenesis[407-414]. There is a close interaction between AMPK and AKT on the ROS homeostasis via mTOR and FOXO regulation, which is of key importance for cancer cells[415].
In a galactose-induced pseudo-aging mouse model, miR-21 significantly increased, whereas miR-21 knockout mice were resistant to galactose-induced alterations in aging-markers[416]. Of note, treatment of rat spinal cord neurons with hydrogen peroxide, a galactose-induced ROS, upregulates miR-21 expression[417].
Milk exosomal microRNAs
Pasteurized commercial cow milk transfers bioavailable extracellular vesicles (EVs) including MEX and their gene-regulatory miRs[418-423]. There is recent evidence that vigorous heat‐treatment such as ultraheat-treatment (UHT: 135 °C, > 1 s) and boiling (100 °C) of commercial cow milk destroys MEVs and MEX and their miR cargo, including miR‐148a[421,424], whereas pasteurization (72-78 °C, > 15 s) of commercial milk did not affect total MEV numbers and preserved nearly 25%-40% of milk’s total small RNAs, including miR‐148a[424]. Bacterial fermentation of milk also attacks MEX and reduces their miR content, as demonstrated for miR-21 and miR-29b in yogurt cultures[425]. Translational evidence indicates that pasteurized non-fermented cow milk is a stronger promoter of mTORC1 activity compared to fermented milk products[426]. Bovine and human MEX and their miRs resist degradative conditions in the gastrointestinal tract, reach the systemic circulation, and distribute in various tissues[420,427-434]. In fact, increasing evidence presented by studies in humans and animal models supports the view that MEX and their miRs are bioavailable, reach the systemic circulation[420,422,434-437], and modify gene expression of the milk recipient[359,418,437-439]. It has been demonstrated that bovine MEX increased the expression of GRP94[253], which is a key endoplasmic reticulum chaperone enhancing the synthesis of insulin, IGF-1, and
MicroRNA-21
Bovine miR-21 is an abundant signature miR of cow milk[451]. Bovine and human miR-21 exhibit nucleotide sequence homology[452-454]. Plasma concentrations of Bos taurus (bta)-miR-21-5p was > 100% higher 6 h after commercial cow milk consumption of healthy human volunteers than before milk consumption, strengthening the bioavailability of milk-derived miRs in human milk consumers[422]. Sadri et al.[437] showed that, after oral gavage of fluorophore-labeled bovine MEX to pregnant mice, miR-21-5p and miR-30d accumulated in placenta and embryos. Experimental evidence provided in murine models demonstrates that oral uptake of bovine MEX results in MEX distribution in various tissues and organs[420,437,455]. MEX miR-21 most likely also affects the prostate gland, where it may target IGFBP3, PTEN, FoxO1, FoxO3, PDCD4, etc., enhancing IGF-1-PI3K-AKT-mTORC1 signaling.
Marquez et al.[456] established post-transcriptional regulation of SMAD7 by miR-21. miR-21 is a key negative regulator of SMAD7 and directly interacts with the 3’UTR of SMAD7 mRNA[456,457]. Importantly,
MicroRNA-148a
miR-148a represents the most abundant miR in cow milk, milk fat, EVs, and MEX[418,436,451,464-467]. miR-148a is highly conserved among mammals[418,468] and has been identified as a domestication gene of dairy cows, enhancing milk yield[469,470]. Human and bovine milk miR-148a nucleotide sequences are identical[418,468,471,472]. Milk-derived bovine miR-148a is thus able to affect gene expression of human milk consumers (cross-species communication)[473]. DNMT1 is a major target of miR-148a[109,110], which explains MEX-mediated suppression of DNMT1 expression[418,438], a pivotal postnatal mechanism modifying epigenetic regulation activating mTORC1 signaling[153,439,443,474]. In fact, DNMT1 inhibition upregulates the expression of the transcription factor nuclear factor erythroid 2-related factor 2 (NRF2)[475], which promotes the expression of mTOR (MTOR)[476]. By targeting the catalytic subunit α1 of AMPK (PRKAA1) as well as the AMPK regulatory subunit γ2 (PRKAG2), miR-148a attenuates the expression AMPK[477,478]. AMPK-mediated phosphorylation of TSC2 and Raptor suppresses mTORC1 activity[315,479]. Importantly, miR-148a inhibits PTEN, the upstream negative regulator of PI3K[106-108], which is downregulated in PCa[22,39]. In addition, miR-148a targets PIK3IP1, the direct negative regulator of PI3K[102]. Thus, milk miR-148a epigenetically augments several checkpoints activating mTORC1 [Figure 2B].
MicroRNA-155 and microRNA-223
miR-155 and miR-223 are dominant immune regulatory miRs of bovine milk[427,428,466,480,481]. miR-155 targets IGFBP3[482]. In synergy with miR-148a, miR-155 also suppresses the expression of PTEN[483]. Both miR-155 and miR-223 suppress the proteasomal degradation mTOR via targeting F-box and WD40 domain protein 7 (FBXW7)[484,485], a critical regulatory checkpoint involved in ubiquitination-dependent degradation of mTOR[486]. Moreover, FBXW7-mediated mTOR degradation cooperates with PTEN in tumor suppression[486]. In addition, FBXW7 promotes the degradation of cyclin E, c-MYC, MCL-1, JUN, NOTCH, and aurora kinase A (AURKA)[487]. In primary PCa, decreased expression of FBXW7 mRNA compared to normal prostate tissues has been detected[488]. Significant overexpression and gene amplification of AURKA and n-MYC have been detected in 40% of neuroendocrine PCa and 5% of PCa, respectively[489].
MicroRNA-125b and microRNA-30d
miR-125b, another abundant miR component of cow milk, resists gastrointestinal digestion[428,465,481]. miR-30d belongs to the top 10 expressed milk miRs when comparing the sequence data of various species including Bos taurus and Homo sapiens[418,436,490,491]. After oral gavage of bovine MEX transfected with fluorophore (IRDye)-labeled miR-30d as well as miR-21 to C57BL/6 mice, these miRs accumulated in murine placenta and embryos[437]. Of note, miR-125b and miR-30d are key inhibitors of TP53, the guardian of the genome[492-494]. In fact, increased expression of miR-125b enhances PCa growth[495] and attenuates the expression of p14(ARF), modifying p53-dependent and -independent apoptosis in PCa[496]. Loss of p53 function plays a critical role in prostate carcinogenesis, especially in early stage[497]. Bovine MEX miR-125b and miR-30d via targeting TP53 may represent another mechanistic link of milk signaling, enhancing mTORC1 in PCa[498]. Notably, p53 induces the expression of a group of p53 target genes in the IGF-1/AKT/mTORC1 pathway. These gene products are negative regulators of the IGF-1/AKT/mTORC1 pathway in response to stress signals[498]. They are IGFBP3[499], PTEN[500-503], TSC2[500], and AMPK β1[500]. Furthermore, p53 is a key inhibitor of AR expression[504]. Milk miR-125b- and miR-30d-mediated suppression of p53 thus attenuates the activity of crucial negative regulators of androgen and mTORC1 signaling, both related to PCa pathogenesis [Figure 2B][494,498].
Xie et al.[448] demonstrated that porcine MEX miRs reduced the expression of p53 in intestinal epithelial cells. The expression of p53 is primarily controlled by the interaction of mouse double minute 2 (MDM2) and MDM4, which are both negative regulators of p53 expression[505]. MDM4 suppresses p53 transcriptional activity and facilitates p53 proteasomal degradation via binding MDM2’s E3 ligase activity towards p53[505]. Overexpression of MDM4 has recently been detected in PCa tissue and increases with disease progression[506-508]. Recent transcriptomic characterization of MEX isolated from cow, donkey, and goat milk identified MDM4 as a central node protein for all three species, indicating a conserved checkpoint with higher numbers of interconnections[509]. Notably, MEX-mediated attenuation p53 signaling has been implicated to play a role in the pathogenesis of PCa and acne vulgaris[494].
Taken together, an interactive network of milk miRs (miR-21, miR-30d, miR-125b, miR148a, and miR-155) provides an epigenetic regulatory layer that inactivates p53 and activates PI3K-AKT-mTORC1-signaling, which drives PCa carcinogenesis[510]. Of note, miR-30d is a critical osteomiR, promoting RUNX2 expression[511], which is closely linked to RUNX2-mediated bone metastasis in PCa[54,56].
MicroRNA-29b
miR-29b, another prominent miR of commercial cow milk, survives pasteurization and storage[419]. As shown in intestinal epithelial cells, bovine MEX miR-29b is taken up by endocytosis[512]. In a dose-dependent manner, plasma levels of miR-29b increased 6 h after consumption of 0.25, 0.5, and 1.0 L of commercial milk and affected blood monocyte gene expression[359]. In a synergistic fashion with miR-148a- and miR-21-mediated inhibition of DNMT1, miR-29b attenuates DNA methylation via suppression of DNMT3A/B[513-516]. Thus, milk-derived miRs via attenuation of DNA methylation of developmental genes such as INS and IGF1 enhance their expression, resulting in increased mTORC1 activity[443].
miR-29b suppresses the catabolism of BCAAs via targeting the mRNA for the dihydrolipoamide branched-chain transacylase (DBT). DBT is the E1α-core subunit of branched-chain α-ketoacid dehydrogenase (BCKD), which degrades BCAAs[517]. The activity of BCKD is regulated by BCKD kinase, which phosphorylates two serine residues in the E1α subunit and thereby inhibits BCKD. Insulin is a known stimulator of BCKD kinase expression, thereby inhibiting BCKD, which results in increased cellular levels of BCAAs[518-523]. In synergy with insulin, MEX miR-29b inhibits the oxidative catabolism of BCAAs required for mTORC1 activation at both the PI3K-AKT-TSC2-RHEB and the BCAA-RAG-Ragulator-RHEB pathway. Intriguingly, increased expression of PCa EV-associated miR-29b-3p could be detected in PCa patients compared to controls[524].
Of importance, it has been shown in osteoblasts that miR-29b induces protein and mRNA expression of RUNX2[525]. Baier et al.[359] demonstrated increased expression of RUNX2 in peripheral blood mononuclear cells of healthy volunteers 6 h after consumption of commercial cow milk. Thus, milk-induced PI3K-AKT signaling with AKT-mediated nuclear extrusion of FoxO1[53] as well as MEX miR-29b-induced RUNX2 expression and enhanced RUNX2 activity[525] provide potential mechanisms for the promotion of PCa bone metastasis[54-56,358].
Milk consumption via upregulation of RUNX2[359] may not only stimulate skeletal development[459,526,527] and linear growth in childhood[261-264] but also promote bone metastasis of PCa [Figure 2B][528].
Bovine milk and meat factors
Small circular single-stranded DNA (ssDNA) sequences have been detected in commercial milk[529-533]. These replication-competent bovine meat and milk factors (BMMF1 and BMMF2) are a specific class of infectious agents spanning between bacterial plasmid and circular ssDNA viruses with similarities to the genomic structure of hepatitis deltavirus[534]. BMMF Rep protein has been found in close vicinity of CD68+ macrophages in the interstitial lamina propria adjacent to colorectal cancer tissues[535]. BMMF1 DNA was isolated from the same tissue regions. Compared to cancer-free controls, Rep and CD68+ exhibited increased expression in peritumor cancer tissues[535]. At present, no experimental data on BMMFs in PCa tissue are available[536].
Aflatoxins
Ruminants metabolize aflatoxin B1 (AFB1) ingested by contaminated food to aflatoxin M1 (AFM1). AFM1 is the hydroxylated mycotoxin that is excreted into milk[537-540]. The increase of AFM1 concentrations in milk of maize-fed cows due to the climate change is a matter of concern[541]. The International Agency for Research on Cancer classified AFB1 and AFM1 as human carcinogens of group 1[542,543]. AFM1 is relatively stable during pasteurization, storage, and processing[544-546]. Scaglioni et al.[547] analyzed AFB1 and AFM1 concentrations of pasteurized and UHT milk and found levels for both aflatoxins in the range of 0.7-
Smith et al.[548] demonstrated a rapid uptake of AFB1 by the rat and dog prostate. Notably, the expression of androgen-inducible aldehyde reductase, a member of the aldo-keto reductase superfamily, exhibits 80% amino acid sequence homology with rat aflatoxin B1 aldehyde reductase and is associated with growth-related processes in regrowing rat prostate after androgen replacement[549]. Aflatoxin B1 aldehyde reductases, specifically the NADPH-dependent aldo-keto reductases of rat (AKR7A1) and human (AKR7A2), are known to metabolize the AFB1 dihydrodiol by forming AFB1 dialcohol[550,551]. Milk-derived aflatoxins may thus modify aldo-keto reductase activities, which are in the focus of recent PCa research[552-554]. Furthermore, it has been demonstrated in lung cancer cell lines that AFB1 upregulates insulin receptor substrate 2; induces SRC, AKT, and ERK1/2 phosphorylation; and stimulates cancer cell migration, which was inhibited by saracatinib[555], a kinase inhibitor under investigation in the treatment of PCa[556-559]. Thus, milk-derived aflatoxins may amplify SRC- and AKT-mediated prostate carcinogenesis. Table 3 summarizes all milk-derived signals that increase PI3K-AKT-mTORC1 signaling.
Overactivated PI3K-AKT-mTORC1 signaling in PCa compared to milk signaling
| Factor | Prostate cancer | Ref. | Milk-derived signaling | Ref. |
| GH | GH and GHR is expressed in PCa tissue; activates PCa cell proliferation and PI3K; induces hepatic IGF-1 secretion; induces AR splice variant 7 | [215-218,228] | Milk consumption increases GH serum levels in children and adults | [212,213] |
| IGF-1 | Systemically and locally increased in PCa | [125-151] | Milk-induced IGF-1 increases serum levels in all age groups, component of bovine milk; activates PI3K-AKT-mTORC1 signaling | [212-213,229-235,237-239] |
| MFG-E8 | Higher expression in PCa and serum exosomes of PCa patients; interacts with αvβ5 integrin | [327,328] | Component of MFG membranes; MFG-E8 mRNA is a cargo of MEX; promotes PI3K-AKT-mTORC1 signaling | [324-327,329,332] |
| Estrogens | ERα is expressed in PCa and promotes PCa progression; increases expression of AR and IGF-1 by E1 in PCa cells | [381-385] | Increased levels of milk estrogens of permanently pregnant dairy cows, especially elevation of E1 | [373] |
| miR-148a | OncomiR of PCa, correlates with Gleason score; targets the PI3K inhibitor PIK3IP1 increasing PI3K activity; inhibits PTEN and DNMT1 expression | [97-100,106-110] | Most abundant miR of bovine milk and MEX surviving pasteurization; identical with human miR-148a; targets DNMT1; enhances expression of INS, IGF1, and TOR and suppresses AMPK activity | [418,421,436,451,454,478,464-467] |
| miR-21 | Biomarker and oncomiR of PCa progession; inhibits PTEN, FOXO1, and SMAD7 expression; increases RUNX2 expression | [69,70,78-95,456-458] | Signature miR of cow milk and MEX; increases in serum after milk consumption; identical with human miR-21; bovine MEX miR-21 accumulates in peripheral murine tissues | [420,422,438,451] |
| miR-125b | Enhances PCa growth, suppresses p53, the inhibitor of AR and mTORC1 | [492-495] | Dominant and resistant miR of commercial milk | [428,465,481] |
| miR-30d | OncomiR of PCa promoting RUNX2 expression; suppresses p53, the negative regulator of AR and mTORC1 | [494,498,510,511] | Belongs to the top 10 miRs of bovine milk; component of bovine MEX accumulating in distant murine tissues afer oral gavage | [418,436,437,490,491] |
| miR-155 | Reduced expression of FBXW7 in PCa | [487,488] | miR component of cow milk; targets PTEN and FBXW7 and thereby activates mTORC1 signaling | [483,484,486] |
| miR-223 | Reduced expression of FBXW7 in PCa | [487,488] | miR component of cow milk; targets FBXW7 and attenuates proteasomal mTOR and oncogen degradation | [485,486] |
| miR-29b | Marker of EVs of PCa | [524] | Dose-dependent increase in serum 6 h after milk consumption; attenuates BCAA catabolism via targeting DBT; induces RUNX2 expression | [359,517,525] |
| BCAA | PCa cells are leucine addicts and increase intracellular leucine content via AR-mediated upregulation of LATs for mTORC1-driven growth | [46,49] | Compared to other protein sources, whey and casein proteins contain high amounts of leucine as well as glutamine, critical amino acids activating mTORC1 | [277-298] |
| Palmitic acid | PCa cells exhibit high uptake of palmitic acid; palmitic acid intake correlates with PCa risk | [321,322] | Palmitic acid is the most abundant saturated fatty acid of milk triacylglycerols; palmitic acid activates mTORC1 at the lysosome; MFG-E8 promotes fatty acid uptake and deposition | [311,318,319,333] |
| Aflatoxin (M, B1) | Rapid uptake of AFB1 in rat and dog prostate | [548] | Commercial milk is contaminated with aflatoxins; AFM1 levels rise in maize-fed cows | [537-541,548] |
| mTORC1 | Upregulated in PCa; involved in PCa intiation and progression; phosphorylation of ERα activating ER target gene expression | [57-62,393] | Milk via insulin/IGF-1 signaling and milk-derived BCAAs (leucine) activates mTORC1 further augmented by milk-derived miRs, especially miR-148a and miR-21 | [20,21,154] |
| FoxO1 | Inactivated by increased PI3K-AKT signaling, functions as nuclear cosuppressor of AR | [41-44,53] | Nuclear FoxO1 inactivation by milk consumption via AKT-mediated phosphorylation and nuclear extrusion | [609,611] |
| Phytanic acid | Associated with PCa risk; increases γ-secretase activity, which cleaves CD44 that interacts with RUNX2 | [339-342,353,362] | Branched-chain fatty acid of milk, increased by organic milk production | [339-342] |
| RUNX2 | Increased RUNX2 expression; bone metastasis, increases PSA transcription; is suppressed by nuclear FoxO1 | [53,55,56,352] | Increased 31% in blood mononuclear cells 6 h after milk consumption | [359] |
| DNMT1 | Reduced expression associated with EMT and cancer stem cell phenotype | [105] | Bovine MEX miR-148a suppresses DNMT1 expression | [418,438,443] |
| Calcium | Thought to be involved in PCa pathogenesis (early studies) | [115-119,124] | Major mineral of milk; questionable influence of dairy calcium on the prostate as circulatory calcium levels are maintained within close limits | [116] |
MILK’S IMPACT ON DEVELOPMENTAL PERIODS OF PCa
Fetal prostate growth
Prostate organogenesis includes organ specification, epithelial budding, branching morphogenesis, canalization, and cytodifferentiation[560]. Activated AR‐positive murine epithelium initiates budding at E17.5. Ductal expansion and branching continues during postnatal development, leading to formation of a fully functional prostate by puberty[561-563].
IGF-1 plays a key role in fetal prostate development[561]. Prostate glands from 44-day-old IGF-1-deficient mice were smaller than those from wild-type mice and exhibited fewer terminal duct tips and branch points and deficits in tertiary and quaternary branching, indicating a specific impairment in gland structure[564]. Furthermore, IGF-1 controls prostate fibromuscular development of the prostatic gland, whereas IGF-1 inhibition prevented both fibromuscular and glandular development in eugonadal mice[565]. Castration rapidly decreased local IGF-1 levels and inhibited its effects in the ventral prostate in mice, whereas local injection of IGF-1 increased vascular density and epithelial cell proliferation in intact mice but had no effect in castrated animals[566]. Studies using mice with liver-specific IGF-1 knockout have demonstrated that liver-derived IGF-1, constituting a major part of circulating IGF-1, is an important endocrine factor involved in a variety of physiological and pathological processes[567]. Notably, locally derived IGF-1 cannot replace liver-derived IGF-1 for the regulation of a large number of other parameters including GH secretion, cortical bone mass, and prostate size[567].
IGF-1 is a regulator of intrauterine growth, and circulating concentrations are reduced in intrauterine growth-restricted fetuses[568]. During fetal life, IGF-1 is mostly secreted by the placenta[569]. Positive correlations among birthweight (BW), gestational age, and umbilical cord serum IGF-1 levels have been reported[569,570]. Thus, increased BW may represent systemic fetal exposure to IGF-1, which may affect prostate branching morphogenesis that may be over-stimulated by maternal milk intake raising maternal systemic IGF-1 levels[231-236].
Downstream PI3K-AKT-mTORC1 signaling plays a crucial role for prostatic morphogenesis[571]. PI3K/mTORC1 signaling is necessary for prostatic epithelial bud invasion of surrounding mesenchyme. The balance of PI3K and downstream mTORC1/C2 activity as a critical regulator of prostatic epithelial morphogenesis[571].
RUNX2 plays an important role in branching morphogenesis of the prostate[572]. IGF-1-PI3K-AKT-mediated nuclear extrusion of FoxO1 enhances nuclear RUNX2 activity[53]. miR-21, which is transferred via MEX, accumulates in the placenta and fetal tissues[420,437] and may further augment RUNX2-dependent prostate morphogenesis and stem cell activation[572]. RUNX2 was detected during early prostate development (E16.5). In adult mice, RUNX2 was expressed in basal and luminal cells of ventral and anterior lobes. Prostate-selective deletion of RUNX2 severely inhibited growth and maturation of tubules in the anterior prostate and reduced expression of stem cell markers and prostate-associated genes[572]. Milk-induced changes of growth trajectories during pregnancy and fetal development via IGF-1 and miR-21 signaling may thus disturb early prostate morphogenesis, increasing subsequent PCa risk in adult life. Increased cow milk intake during the first trimester of pregnancy has recently been positively associated with general and abdominal visceral fat mass and lean mass at the age of 10 years[573].
BW and PCa risk
BW, an indicator of fetal growth, is related to placental weight[574] and has been identified as a risk factor of PCa[575-578]. Compared with twins with a BW of 2500-2999 g, the hazard ratio (95%CI) for twins with a higher BW (≥ 3000 g) corresponded to 1.22 (0.94-1.57). In analyses within twin pairs, in which both twins had a BW of ≥ 2500 g, a 500 g increase in BW was associated with an increased risk of PCa within dizygotic twin pairs [odds ratio (OR) = 1.41, 95%CI: 1.02-1.57)], but not within monozygotic twin pairs (OR = 1.06, 95%CI: 0.61-1.84)[578]. Especially, BW ≥ 4250 g was associated with significantly higher PCa incidence [62% (CI: 4%-151%)] and PCa mortality [82% (CI: 3%-221%)] than BW 3001-4249 g[576]. High BW is related to increased risks of total and aggressive/lethal PCa[577], underlining that intrauterine exposures may enhance PCa risk and course. In fact, the Malmö Diet and Cancer Study reported a protective effect of lower BW on risk of total and aggressive PCa[579].
Remarkably, maternal milk consumption but not the intake of fermented milk products (cheese) was associated with fetal weight gain and higher BW[580,581], as subsequently confirmed by systematic reviews[582-584]. In contrast to fermented milk with degraded MEX[425], raw and pasteurized milk delivers MEX and MEX miR-21[418,422,424,451], which in murine models reach the placenta and peripheral tissues[420,437] and have been related with placental weight and fetal overgrowths (macrosomia)[585,586]. Milk protein-derived essential amino acids and MEX-derived miRs, especially miR-21 and miR-148a, promote mTORC1 activity. Increased trophoblast mTORC1 activity determines placental-fetal transfer of amino acids and glucose and thus fetal growth and BW[587-591]. Of note, mTORC1 signaling regulates the expression of trophoblast genes involved in ribosome and protein synthesis, mitochondrial function, lipid metabolism, nutrient transport, and angiogenesis, representing novel links between mTORC1 signaling and multiple placental functions critical for fetal growth and development[592]. It is worth mentioning that other nutritional factors unrelated to milk intake, such as total animal protein intake during pregnancy as well as other nutritional factors such as ω-3 fatty acid intake and folic acid supplementation, have an influence on BW, which are not discussed in this review. Taken together, epidemiological and translational evidence supports the view that maternal milk consumption during pregnancy modifies fetal mTORC1-driven growth trajectories that determine BW and may also affect early prostate development and morphogenesis.
Height during puberty and PCa risk
According to the Copenhagen School Health Records Register and the Danish Cancer Registry, childhood height at age 13 years showed a positive association with PCa-specific mortality[593-596]. The PCa-promoting effect of height at 13 years was not entirely dependent on adult height, suggesting different modes of action[596]. These findings implicate late childhood and adolescence are critical exposure windows of interest that underlie the association between height and PCa[593]. According to the Longitudinal Studies of Child Health and Development, fat and animal protein intake during childhood was positively related with height at age 13 and adult height[597]. Notably, an earlier age at peak height velocity was associated with a diet high in fat and animal protein and low in vegetable protein during childhood[597]. Childhood diet and accelerated growth thus influence earlier pubertal timing and taller attained height in males, supporting their contribution in the pathogenesis of PCa[597].
Notably, The NHANES 1999-2002 study reported that consumption of milk, in contrast to other dairy products, is related to height among US preschool children[598]. The frequency of milk consumption and milk intake were identified as significant predictors of height at 12-18 years[599]. Almon et al.[600], who explored the potential relationship among the LCT (lactase) C>T-13910 polymorphism, milk consumption, and height in a sample of Swedish preadolescents and adolescents, reported a positive association between milk consumption and height in preadolescents and adolescents. In fact, increased consumption of cow milk, which leads to higher levels of IGF-1 in circulation, promotes increased velocity of linear growth[261,264].
In a population-based cohort of 8894 men born between 1907 and 1935, Torfadottir et al.[168] studied the effects of early-life residency in Iceland and differences in milk intake in relation to the risk of PCa later in life. Remarkably, a 3.2-fold risk of advanced PCa was related to daily milk consumption in adolescence (vs. less than daily), but not consumption in midlife or currently. Accordingly, frequent milk intake especially in adolescence increases risk of advanced PCa[168]. Lan et al.[10] recently analyzed dietary data of the NIH-AARP Diet and Health Study from 162,816 participants over a follow-up period of 14 years to investigate potential associations for milk, cheese, ice cream, total dairy, and calcium intake at ages 12-13 years with incident total (n = 17,729), advanced (n = 2348), and fatal PCa (n = 827). Their findings also support the contribution of milk intake during adolescence for increased risk of PCa [Figure 3][10].
Remarkably, milk consumption during adolescence has also been associated with acne vulgaris, the most common inflammatory skin disease of adolescents in Western civilizations[601-607]. Notably, acne risk is related with height at puberty[608]. A cross-sectional population-based study on 6200 boys showed that 12-15-year-old boys with acne were taller and heavier than those without acne[608]. In analogy to PCa, both androgens and IGF-1 induce the sebaceous gland disease acne vulgaris, which is associated with increased glandular mTORC1 activity[609-611]. It is thus not surprising that an epidemiological association between height and acne during adolescence and PCa during adult life has been observed [Figure 4][612-614].
DISPUTABLE STUDIES FOR MILK-PCa RISK EVALUATION
Cell cultures
There are several examples of investigations that apply questionable study designs to analyze the relationship between milk consumption and PCa risk. Tate et al.[615] added cow milk to LNCaP cells and observed an increased growth rate of over 30%. However, under physiological conditions, a prostate cell will never be exposed to whole milk. Transferable factors of milk, such as BCAAs, IGF-1, estrogens, aflatoxins, exosomal MFG-E8, and exosomal miRs, may eventually reach the prostate and may accumulate during persistent milk consumption.
Park et al.[616] exposed PCa cells (LNCaP and PC3) and immortalized normal prostate cells (RWPE1) with either 0.1 or 1 mg/mL of α-casein and total casein extracted from bovine milk. Whereas α-casein and total casein did not affect the proliferations of RWPE1 cells, PC3 and LNCaP cells showed a significant but IGF-1-independent increase in cell proliferation.
It is known that LNCaP and PC-3 cells take up leucine in a PI3K-AKT-dependent manner[50]. Casein hydrolysis may provide abundant leucine for PCa proliferation. Notably, total and α-casein contain high amounts of leucine of 9.2 and 7.9 g/100g protein, respectively[617]. Kim et al.[618] studied gene expression profiles of PC3 cells after exposure to α-casein and showed activated PI3K/AKT/mTORC1 signaling. Under in vivo conditions, however, α-casein will never reach the prostate and PCa tissue, whereas α-casein-derived BCAAs after intestinal hydrolysis and uptake into the blood circulation may affect PCa via BCAA-mTORC1 signaling.
Animal experiments
Bernichtein et al.[619] performed an interventional animal study using two mouse models of fully penetrant genetically induced prostate tumorigenesis that were investigated at the stages of benign hyperplasia (probasin-Prl mice, Pb-Prl) or pre-cancerous prostatic intraepithelial neoplasia lesions (KIMAP mice). They reported that mice were fed high milk diets (skim or whole milk) for 15-27 weeks depending on the kinetics of prostate tumor development in each model. They reported that that high milk consumption did not promote progression of existing prostate tumors when assessed at early stages of tumorigenesis. Unfortunately, these investigators did not use regular commercial cow milk, but they instead exposed their animals to milk protein powder re-suspended with water[619]. It is thus conceivable that the impact of milk EVs and MEX and their miR signaling was neglected by the selected feeding design. Retrospectively, they missed the complexity of milk signaling and thus provided a questionable conclusion.
Mendelian randomization studies
Larsson et al.[620] investigated the potential causal associations of milk consumption with the risk of PCa using genetic variants (rs4988235 or rs182549) near the LCT gene as proxies for milk consumption and reported no overall association between genetically predicted milk consumption and PCa (OR = 1.01, 95%CI: 0.99-1.02, P = 0.389). However, there was moderate heterogeneity among estimates from different data sources (I2 = 54%). In contrast, a positive association was observed in the FinnGen consortium (OR = 1.07, 95%CI: 1.01-1.13, P = 0.026), which was more homogenous than the two other European populations [UK Biobank cohort and Prostate Cancer Association Group to Investigate Cancer Associated Alterations in the Genome (PRACTICAL) consortium]. Notably, per capita milk consumption in Finland (100.8 kg in 2020) is the highest in Europe[621]. The approximations of milk intake were based on data of rs4988235 (LCT-12910C>T) of a sub-cohort of 12,722 participants of the European Prospective Investigation into Cancer and Nutrition-InterAct study[622]. The median milk consumption was 162 g/day (25th-75th percentile, 37-300 g/day) and each additional milk intake increasing allele of rs4988235 was associated with an increase in milk consumption of 17.1 g/day (P = 2 × 10-7). Unfortunately, rs4988235 was not available in the FinnGen consortium, and instead a proxy SNP (rs182549) in complete linkage disequilibrium was used. A further limitation of Mendelian randomization studies is the fact that they do not present certain data on the real daily amount of consumed milk, milk’s thermal processing method used in the analyzed study cohorts, and the period of milk consumption during individual life periods as well as the potential impact of maternal milk consumption on fetal prostate development, a critical period for nutritional epigenetic programming of non-communicable diseases and non-hereditable genotypes[445,623-624].
Meta-analyses and umbrella reviews
Epidemiological studies are also afflicted with severe insufficiencies, as no study reported in the literature paid attention to the thermal processing of milk (pasteurization versus UHT), which significantly modifies the survival and biological activity of milk EVs and MEX and their miRs[421,424,426]. Meta-analyses of meta-analyses (so-called umbrella reviews[625]) mixing cohorts with low milk intake (Asian people) and cohorts with high milk intake (US citizens and Europeans) further modified by different types of thermal milk processing (predominant UHT processing in Southern European countries and preferential pasteurization in Northern European countries) are very questionable scientific approaches from the point of milk’s physiology. Furthermore, no study considered the complete spectrum of vulnerable periods of milk intake during lifetime exposure on prostate tumorigenesis (fetal period, childhood, puberty with final prostate gland differentiation, and adulthood) [Figure 3]. Nevertheless, it is astonishing that, despite these variances and insufficiencies, the majority of dairy industry-independent meta-analyses and prospective studies were able to relate milk consumption with an increased risk of PCa[10,12,13,14,168-175], whereas a recent review series sponsored by the Interprofessional Dairy Organization (INLAC) of Spain concluded that milk and dairy product consumption is not associated with increased all-cause mortality[626] and is not associated with an increased risk of PCa[627].
CONCLUSION
As shown in this review, milk consumption provides a symphony of signals activating PI3K-AKT-mTORC1 and synergistically operates on overactivated PI3K-AKT-mTORC1 signaling pathways of PCa[21,154]. There is no monocausal agent such as calcium, IGF-1, estrogens, BMMFs, or other single compounds that exclusively promote cancer cell growth and PCa development. However, the molecular crosstalk of IGF-1, androgens, estrogens, and milk-derived miRs functionally converge with well-characterized genetic and epigenetic deviations of PI3K-AKT-mTORC1 signal transduction in PCa[22,23,63-68].
Practices of veterinary medicine and dairy research to increase milk yield via holding dairy cows in permanent pregnancy (increase in milk estrogens)[373-377] and selection for high-yield dairy cows (increase of milk miR-148a)[469,470] combined with the induction of pasteurization and refrigeration technology changed the magnitude and biological character of milk-derived signals compared to ancient times, when the total amount and type of dairy intake were predominantly fermented milk products (cheese, yogurt, and kefir)[426].
Pasteurization of milk was introduced as an unproven technology without prior scientific information of its long-term effects on human health. Pasteurization combined with refrigeration allowed the large-scale introduction of milk’s epigenetic signaling machinery into the human food chain, recently promoted as a nutritional and therapeutic opportunity[628]. In contrast to UHT processing of milk, milk EV-derived miRs survive pasteurization, as recently confirmed[629]. We are very concerned that food augmentation with oncogenic MEX-derived miRs will further promote milk-related cancers such as PCa and breast cancer[450]. From the medical point of view, we claim to remove milk’s EVs and MEX from the human food chain to avoid their oncogenic effects[450].
Cow milk consumption in Western societies is a lifelong exposure beginning during fetal life. The fact that maternal milk consumption promotes fetal growth[580-583] implies early effects on prostate development and morphogenesis. In contrast to fermented milk (cheese), only maternal milk intake is correlated with BW[580-583] and increased risk of PCa[10]. It is thus of critical concern that webpages of medical societies such as the American College of Obstetricians and Gynecologists[630] and John Hopkins Medicine[631] still promote milk intake during pregnancy and do not differentiate between the biological effects of milk and those of fermented milk products on fetal development. The next vulnerable window for milk-mediated disturbances is puberty, the period of final morphogenesis of the prostate. Milk-mediated over-stimulation of the sebaceous gland (acne vulgaris), skeletal growth (linear overgrowth), and invisible and undetected changes of the prostatic gland (the potential seeding period of PCa) occur apparently simultaneously during puberty when young men consume milk, especially when fortified with whey protein concentrates combined with androgen abuse to increase muscle mass and physical appearance[632]. In concert with obstetricians and gynecologists, pediatricians recommend milk intake for children and federal governments support school milk consumption[633]. According to Agostoni et al.[634], a maximum daily intake of 500 mL cow milk should be provided to children above the age of 12 months. Although these authors appreciated milk-induced increases of IGF-1 and linear growth, they neglected associations with noncommunicable diseases[634]. Puberty with the final differentiation of the prostatic gland appears to be a very vulnerable life period for milk’s impact on PCa pathogenesis, a critical issue that is only addressed in two epidemiological studies[10,168] but no clinical study.
For adult men, further continuous milk consumption is recommended by orthopedics as a valuable source of calcium for the putative prevention of bone loss[161,635,636]. Thus, important disciplines of Western medicine promote milk consumption during lifetime with very restricted views to the demands of their own specialty. There is no epidemiological study that provides data for milk intake over all vulnerable periods of life. There is no epidemiological study paying attention to the thermal processing of milk, which affects the bioavailability of milk EVs. Very recent evidence in rodent models demonstrates that bovine milk EVs promote cancer metastasis[637]. Further studies of the protooncogene MDM4 as a central node of bovine MEX interconnectivity may be promising for a deeper understanding of milk’s impact on PCa pathogenesis. We hope that our review stimulates urologic oncology to address these questions in future research to prevent epidemic PCa.
DECLARATIONS
AcknowledgmentsThe authors thank Claus Leitzmann, University of Giessen, Germany and Harald zur Hausen, German Cancer Reseach Center (DKFZ) for constructive discussions of milk’s impact on human health.
Authors’ contributionsConception and design: Melnik BC
Administrative and scientific support: John SM, Weiskirchen R, Schmitz G
Collection and assembly of data: Melnik BC, Schmitz G
Data analysis and interpretation: Melnik BC, Schmitz G, Weiskirchen R, John SM
Manuscript writing: Melnik BC
Final approval of the manuscript: Melnik BC, John SM, Weiskirchen R, Schmitz G
Availability of data and materialsData in this review were derived from searches of the PubMed database.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Ferlay J, Ervik M, Lam F, et al. Global cancer observatory: cancer today. Lyon, France: International Agency for Research on Cancer. Available from: https://gco.iarc.fr/today/data/factsheets/cancers/27-Prostate-fact-sheet.pdf [Last accessed on 17 Dec 2021].
2. Culp MB, Soerjomataram I, Efstathiou JA, Bray F, Jemal A. Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol 2020;77:38-52.
3. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209-49.
4. Gupta S, Gupta A, Saini AK, Majumder K, Sinha K, Chahal A. Prostate cancer: how young is too young? Curr Urol 2017;9:212-5.
5. Lin PH, Aronson W, Freedland SJ. Nutrition, dietary interventions and prostate cancer: the latest evidence. BMC Med 2015;13:3.
7. Matsushita M, Fujita K, Nonomura N. Influence of diet and nutrition on prostate cancer. Int J Mol Sci 2020;21:1447.
8. Lin PH, Aronson W, Freedland SJ. An update of research evidence on nutrition and prostate cancer. Urol Oncol 2019;37:387-401.
9. Leslie SW, Soon-Sutton TL, Sajjad H, Siref LE. Prostate cancer. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021.
10. Lan T, Park Y, Colditz GA, et al. Adolescent dairy product and calcium intake in relation to later prostate cancer risk and mortality in the NIH-AARP Diet and Health Study. Cancer Causes Control 2020;31:891-904.
11. Ganmaa D, Li X, Qin L, Wang P, Takeda M, Sato A. The experience of Japan as a clue to the etiology of testicular and prostatic cancers. Med Hypotheses 2003;60:724-30.
12. Qin LQ, Xu JY, Wang PY, Kaneko T, Hoshi K, Sato A. Milk consumption is a risk factor for prostate cancer: meta-analysis of case-control studies. Nutr Cancer 2004;48:22-7.
13. Qin LQ, Xu JY, Wang PY, Tong J, Hoshi K. Milk consumption is a risk factor for prostate cancer in Western countries: evidence from cohort studies. Asia Pac J Clin Nutr 2007;16:467-76.
14. Sargsyan A, Dubasi HB. Milk consumption and prostate cancer: a systematic review. World J Mens Health 2021;39:419-28.
15. Our world in data. Available from: https://ourworldindata.org/grapher/per-capita-milk-consumption [Last accessed on 17 Dec 2021].
16. Milchindustrie-Verband e.V: Deutschland: Pro-Kopfverbrauch von Milchprodukten. Available from: https://milchindustrie.de/wp-content/uploads/2020/04/ProkopfDeutschland_Mopro_2013-2019x_Homepage.pdf [Last accessed on 17 Dec 2021].
17. Ridder M. Statista: per capita consumption of milk in Sweden 2008-2018. Available from: https://www.statista.com/statistics/557618/per-capita-consumption-of-milk-in-sweden/ [Last accessed on 17 Dec 2021].
18. Statista. Per capita consumption of fluid milk products in the United States from 2000 to 2020 (in pounds)*. Available from: https://www.statista.com/statistics/184240/us-per-capita-consumption-of-fluid-milk- products/ [Last accessed on 17 Dec 2021].
19. Statista. Per capita milk and dairy product consumption in China from 2013 to 2020. Available from: https://www.statista.com/statistics/1098497/china-per-capita-milk-dairy-consumption/ [Last accessed on 17 Dec 2021].
20. Melnik BC. Milk-a nutrient system of mammalian evolution promoting mTORC1-dependent translation. Int J Mol Sci 2015;16:17048-87.
21. Melnik BC, John SM, Carrera-Bastos P, Cordain L. The impact of cow's milk-mediated mTORC1-signaling in the initiation and progression of prostate cancer. Nutr Metab (Lond) 2012;9:74.
22. Shorning BY, Dass MS, Smalley MJ, Pearson HB. The PI3K-AKT-mTOR pathway and prostate cancer: at the crossroads of AR, MAPK, and WNT signaling. Int J Mol Sci 2020;21:4507.
23. Wang G, Zhao D, Spring DJ, DePinho RA. Genetics and biology of prostate cancer. Genes Dev 2018;32:1105-40.
24. Carver BS, Chapinski C, Wongvipat J, et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 2011;19:575-86.
25. Crumbaker M, Khoja L, Joshua AM. AR signaling and the PI3K pathway in prostate cancer. Cancers (Basel) 2017;9:34.
26. Pearson HB, Li J, Meniel VS, et al. Identification of Pik3ca mutation as a genetic driver of prostate cancer that cooperates with pten loss to accelerate progression and castration-resistant growth. Cancer Discov 2018;8:764-79.
27. Chen H, Zhou L, Wu X, et al. The PI3K/AKT pathway in the pathogenesis of prostate cancer. Front Biosci (Landmark Ed) 2016;21:1084-91.
28. Turnham DJ, Bullock N, Dass MS, Staffurth JN, Pearson HB. The PTEN conundrum: how to target PTEN-deficient prostate cancer. Cells 2020;9:2342.
29. Cairns P, Okami K, Halachmi S, et al. Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res 1997;57:4997-5000.
30. Suzuki H, Freije D, Nusskern DR, et al. Interfocal heterogeneity of PTEN/MMAC1 gene alterations in multiple metastatic prostate cancer tissues. Cancer Res 1998;58:204-9.
31. Wang SI, Parsons R, Ittmann M. Homozygous deletion of the PTEN tumor suppressor gene in a subset of prostate adenocarcinomas. Clin Cancer Res 1998;4:811-5.
32. Rudge SA, Wakelam MJ. Phosphatidylinositolphosphate phosphatase activities and cancer. J Lipid Res 2016;57:176-92.
33. Jamaspishvili T, Berman DM, Ross AE, et al. Clinical implications of PTEN loss in prostate cancer. Nat Rev Urol 2018;15:222-34.
34. Geybels MS, Fang M, Wright JL, et al. PTEN loss is associated with prostate cancer recurrence and alterations in tumor DNA methylation profiles. Oncotarget 2017;8:84338-48.
35. McMenamin ME, Soung P, Perera S, Kaplan I, Loda M, Sellers WR. Loss of PTEN expression in paraffin-embedded primary prostate cancer correlates with high Gleason score and advanced stage. Cancer Res 1999;59:4291-6.
36. Taylor BS, Schultz N, Hieronymus H, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010;18:11-22.
37. Grasso CS, Wu YM, Robinson DR, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012;487:239-43.
38. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015;161:1215-28.
39. Armenia J, Wankowicz SAM, Liu D, et al. PCF/SU2C International Prostate Cancer Dream Team. The long tail of oncogenic drivers in prostate cancer. Nat Genet 2018;50:645-51.
40. Abida W, Cyrta J, Heller G, et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc Natl Acad Sci U S A 2019;116:11428-36.
41. Liu P, Li S, Gan L, Kao TP, Huang H. A transcription-independent function of FOXO1 in inhibition of androgen-independent activation of the androgen receptor in prostate cancer cells. Cancer Res 2008;68:10290-9.
42. Ma Q, Fu W, Li P, et al. FoxO1 mediates PTEN suppression of androgen receptor N- and C-terminal interactions and coactivator recruitment. Mol Endocrinol 2009;23:213-25.
43. Bohrer LR, Liu P, Zhong J, et al. FOXO1 binds to the TAU5 motif and inhibits constitutively active androgen receptor splice variants. Prostate 2013;73:1017-27.
44. Zhao Y, Tindall DJ, Huang H. Modulation of androgen receptor by FOXA1 and FOXO1 factors in prostate cancer. Int J Biol Sci 2014;10:614-9.
45. Yan Y, Huang H. Interplay among PI3K/AKT, PTEN/FOXO and AR signaling in prostate cancer. Adv Exp Med Biol 2019;1210:319-31.
46. Wang Q, Bailey CG, Ng C, et al. Androgen receptor and nutrient signaling pathways coordinate the demand for increased amino acid transport during prostate cancer progression. Cancer Res 2011;71:7525-36.
47. Millward DJ, Layman DK, Tomé D, Schaafsma G. Protein quality assessment: impact of expanding understanding of protein and amino acid needs for optimal health. Am J Clin Nutr 2008;87:1576S-81S.
48. Souci SW, Fachmann W, Kraut H. Food composition and nutrition tables. 8th revised and extended edition. Volume XXXII. Stuttgart, Germany: MedPharm; 2016. p. 1263.
49. Salisbury TB, Arthur S. The regulation and function of the L-type amino acid transporter 1 (LAT1) in cancer. Int J Mol Sci 2018;19:2373.
50. Zhang BK, Moran AM, Bailey CG, Rasko JEJ, Holst J, Wang Q. EGF-activated PI3K/Akt signalling coordinates leucine uptake by regulating LAT3 expression in prostate cancer. Cell Commun Signal 2019;17:83.
51. Otsuki H, Kimura T, Yamaga T, Kosaka T, Suehiro JI, Sakurai H. Prostate cancer cells in different androgen receptor status employ different leucine transporters. Prostate 2017;77:222-33.
52. Strmiska V, Michalek P, Eckschlager T, et al. Prostate cancer-specific hallmarks of amino acids metabolism: towards a paradigm of precision medicine. Biochim Biophys Acta Rev Cancer 2019;1871:248-58.
53. Zhang H, Pan Y, Zheng L, et al. FOXO1 inhibits Runx2 transcriptional activity and prostate cancer cell migration and invasion. Cancer Res 2011;71:3257-67.
54. Ge C, Zhao G, Li Y, et al. Role of Runx2 phosphorylation in prostate cancer and association with metastatic disease. Oncogene 2016;35:366-76.
55. Akech J, Wixted JJ, Bedard K, et al. Runx2 association with progression of prostate cancer in patients: mechanisms mediating bone osteolysis and osteoblastic metastatic lesions. Oncogene 2010;29:811-21.
56. Kim B, Kim H, Jung S, et al. A CTGF-RUNX2-RANKL axis in breast and prostate cancer cells promotes tumor progression in bone. J Bone Miner Res 2020;35:155-66.
57. Hsieh AC, Liu Y, Edlind MP, et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature 2012;485:55-61.
58. Edlind MP, Hsieh AC. PI3K-AKT-mTOR signaling in prostate cancer progression and androgen deprivation therapy resistance. Asian J Androl 2014;16:378-86.
59. Zabala-letona A, Arruabarrena-aristorena A, Martín-martín N, et al. mTORC1-dependent AMD1 regulation sustains polyamine metabolism in prostate cancer. Nature 2017;547:109-13.
60. Audet-Walsh É, Vernier M, Yee T, et al. SREBF1 activity is regulated by an AR/mTOR nuclear axis in prostate cancer. Mol Cancer Res 2018;16:1396-405.
61. Han Y, Liu C, Zhang D, et al. Mechanosensitive ion channel Piezo1 promotes prostate cancer development through the activation of the Akt/mTOR pathway and acceleration of cell cycle. Int J Oncol 2019;55:629-44.
62. Binal Z, Açıkgöz E, Kızılay F, Öktem G, Altay B. Cross-talk between ribosome biogenesis, translation, and mTOR in CD133+ 4/CD44+ prostate cancer stem cells. Clin Transl Oncol 2020;22:1040-8.
63. Ngollo M, Dagdemir A, Karsli-Ceppioglu S, et al. Epigenetic modifications in prostate cancer. Epigenomics 2014;6:415-26.
64. Liao Y, Xu K. Epigenetic regulation of prostate cancer: the theories and the clinical implications. Asian J Androl 2019;21:279-90.
65. Zhu KC, Lu JJ, Xu XL, Sun JM. MicroRNAs in androgen-dependent PCa. Front Biosci (Landmark Ed) 2013;18:748-55.
66. Eringyte I, Zamarbide Losada JN, Powell SM, Bevan CL, Fletcher CE. Coordinated AR and microRNA regulation in prostate cancer. Asian J Urol 2020;7:233-50.
67. Kanwal R, Plaga AR, Liu X, Shukla GC, Gupta S. MicroRNAs in prostate cancer: Functional role as biomarkers. Cancer Lett 2017;407:9-20.
68. Verma S, Pandey M, Shukla GC, Singh V, Gupta S. Integrated analysis of miRNA landscape and cellular networking pathways in stage-specific prostate cancer. PLoS One 2019;14:e0224071.
69. Folini M, Gandellini P, Longoni N, et al. miR-21: an oncomir on strike in prostate cancer. Mol Cancer 2010;9:12.
70. Ribas J, Lupold SE. The transcriptional regulation of miR-21, its multiple transcripts, and their implication in prostate cancer. Cell Cycle 2010;9:923-9.
71. Yang Y, Guo JX, Shao ZQ. miR-21 targets and inhibits tumor suppressor gene PTEN to promote prostate cancer cell proliferation and invasion: an experimental study. Asian Pac J Trop Med 2017;10:87-91.
72. Lu Z, Liu M, Stribinskis V, et al. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene 2008;27:4373-9.
73. Go H, Jang JY, Kim PJ, et al. MicroRNA-21 plays an oncogenic role by targeting FOXO1 and activating the PI3K/AKT pathway in diffuse large B-cell lymphoma. Oncotarget 2015;6:15035-49.
74. Song W, Li Q, Wang L, Wang L. Modulation of FoxO1 expression by miR-21 to promote growth of pancreatic ductal adenocarcinoma. Cell Physiol Biochem 2015;35:184-90.
75. Shukla S, Shukla M, Maclennan GT, Fu P, Gupta S. Deregulation of FOXO3A during prostate cancer progression. Int J Oncol 2009;34:1613-20.
76. Wang K, Li PF. Foxo3a regulates apoptosis by negatively targeting miR-21. J Biol Chem 2010;285:16958-66.
77. Ribas J, Ni X, Haffner M, et al. miR-21: an androgen receptor-regulated microRNA that promotes hormone-dependent and hormone-independent prostate cancer growth. Cancer Res 2009;69:7165-9.
78. Foj L, Ferrer F, Serra M, et al. Exosomal and non-exosomal urinary miRNAs in prostate cancer detection and prognosis. Prostate 2017;77:573-83.
79. Shin S, Park YH, Jung SH, et al. Urinary exosome microRNA signatures as a noninvasive prognostic biomarker for prostate cancer. NPJ Genom Med 2021;6:45.
80. Danarto R, Astuti I, Umbas R, Haryana SM. Urine miR-21-5p and miR-200c-3p as potential non-invasive biomarkers in patients with prostate cancer. Turk J Urol 2020;46:26-30.
81. Ghorbanmehr N, Gharbi S, Korsching E, Tavallaei M, Einollahi B, Mowla SJ. miR-21-5p, miR-141-3p, and miR-205-5p levels in urine-promising biomarkers for the identification of prostate and bladder cancer. Prostate 2019;79:88-95.
82. Zhou H, Zhu X. MicroRNA-21 and microRNA-30c as diagnostic biomarkers for prostate cancer: a meta-analysis. Cancer Manag Res 2019;11:2039-50.
83. Huang W, Kang XL, Cen S, Wang Y, Chen X. High-level expression of microRNA-21 in peripheral blood mononuclear cells is a diagnostic and prognostic marker in prostate cancer. Genet Test Mol Biomarkers 2015;19:469-75.
84. Zedan AH, Blavnsfeldt SG, Hansen TF, et al. Heterogeneity of miRNA expression in localized prostate cancer with clinicopathological correlations. PLoS One 2017;12:e0179113.
85. Sharma N, Baruah MM. The microRNA signatures: aberrantly expressed miRNAs in prostate cancer. Clin Transl Oncol 2019;21:126-44.
86. Arisan ED, Rencuzogullari O, Freitas IL, et al. Upregulated Wnt-11 and miR-21 expression trigger epithelial mesenchymal transition in aggressive prostate cancer cells. Biology (Basel) 2020;9:52.
87. Guan C, Zhang L, Wang S, et al. Upregulation of microRNA-21 promotes tumorigenesis of prostate cancer cells by targeting KLF5. Cancer Biol Ther 2019;20:1149-61.
89. Mishra S, Lin CL, Huang TH, Bouamar H, Sun LZ. MicroRNA-21 inhibits p57Kip2 expression in prostate cancer. Mol Cancer 2014;13:212.
90. Li T, Li D, Sha J, Sun P, Huang Y. MicroRNA-21 directly targets MARCKS and promotes apoptosis resistance and invasion in prostate cancer cells. Biochem Biophys Res Commun 2009;383:280-5.
91. Mishra S, Deng JJ, Gowda PS, et al. Androgen receptor and microRNA-21 axis downregulates transforming growth factor beta receptor II (TGFBR2) expression in prostate cancer. Oncogene 2014;33:4097-106.
92. Yang CH, Pfeffer SR, Sims M, et al. The oncogenic microRNA-21 inhibits the tumor suppressive activity of FBXO11 to promote tumorigenesis. J Biol Chem 2015;290:6037-46.
93. Reis ST, Pontes-Junior J, Antunes AA, et al. miR-21 may acts as an oncomir by targeting RECK, a matrix metalloproteinase regulator, in prostate cancer. BMC Urol 2012;12:14.
94. Porzycki P, Ciszkowicz E, Semik M, Tyrka M. Combination of three miRNA (miR-141, miR-21, and miR-375) as potential diagnostic tool for prostate cancer recognition. Int Urol Nephrol 2018;50:1619-26.
95. Hatano K, Fujita K. Extracellular vesicles in prostate cancer: a narrative review. Transl Androl Urol 2021;10:1890-907.
96. Szczyrba J, Löprich E, Wach S, et al. The microRNA profile of prostate carcinoma obtained by deep sequencing. Mol Cancer Res 2010;8:529-38.
97. Dybos SA, Flatberg A, Halgunset J, et al. Increased levels of serum miR-148a-3p are associated with prostate cancer. APMIS 2018;126:722-31.
98. Gurbuz V, Sozen S, Bilen CY, Konac E. miR-148a, miR-152 and miR-200b promote prostate cancer metastasis by targeting DNMT1 and PTEN expression. Oncol Lett 2021;22:805.
99. Murata T, Takayama K, Katayama S, et al. miR-148a is an androgen-responsive microRNA that promotes LNCaP prostate cell growth by repressing its target CAND1 expression. Prostate Cancer Prostatic Dis 2010;13:356-61.
100. Jalava SE, Urbanucci A, Latonen L, et al. Androgen-regulated miR-32 targets BTG2 and is overexpressed in castration-resistant prostate cancer. Oncogene 2012;31:4460-71.
101. Hamilton MP, Rajapakshe KI, Bader DA, et al. The landscape of microRNA targeting in prostate cancer defined by AGO-PAR-CLIP. Neoplasia 2016;18:356-70.
102. Zhu Z, He X, Johnson C, et al. PI3K is negatively regulated by PIK3IP1, a novel p110 interacting protein. Biochem Biophys Res Commun 2007;358:66-72.
103. Valdez CD, Kunju L, Daignault S, Wojno KJ, Day ML. The E2F1/DNMT1 axis is associated with the development of AR negative castration resistant prostate cancer. Prostate 2013;73:1776-85.
104. Sengupta D, Deb M, Patra SK. Antagonistic activities of miR-148a and DNMT1: ectopic expression of miR-148a impairs DNMT1 mRNA and dwindle cell proliferation and survival. Gene 2018;660:68-79.
105. Lee E, Wang J, Yumoto K, et al. DNMT1 regulates epithelial-mesenchymal transition and cancer stem cells, which promotes prostate cancer metastasis. Neoplasia 2016;18:553-66.
106. He H, Cai M, Zhu J, et al. miR-148a-3p promotes rabbit preadipocyte differentiation by targeting PTEN. In Vitro Cell Dev Biol Anim 2018;54:241-9.
107. Qingjuan L, Xiaojuan F, Wei Z, et al. miR-148a-3p overexpression contributes to glomerular cell proliferation by targeting PTEN in lupus nephritis. Am J Physiol Cell Physiol 2016;310:C470-8.
108. Jin X, Hao Z, Zhao M, et al. MicroRNA-148a regulates the proliferation and differentiation of ovine preadipocytes by targeting PTEN. Animals (Basel) 2021;11:820.
109. Pan W, Zhu S, Yuan M, et al. MicroRNA-21 and microRNA-148a contribute to DNA hypomethylation in lupus CD4+ T cells by directly and indirectly targeting DNA methyltransferase 1. J Immunol 2010;184:6773-81.
110. Yang A, Sun Y, Gao Y, et al. Reciprocal regulation between miR-148a/152 and DNA methyltransferase 1 is associated with hyperhomocysteinemia-accelerated atherosclerosis. DNA Cell Biol 2017;36:462-74.
111. Kuroda A, Rauch TA, Todorov I, et al. Insulin gene expression is regulated by DNA methylation. PLoS One 2009;4:e6953.
112. Ouni M, Gunes Y, Belot MP, Castell AL, Fradin D, Bougnères P. The IGF1 P2 promoter is an epigenetic QTL for circulating IGF1 and human growth. Clin Epigenetics 2015;7:22.
113. Ouni M, Castell AL, Rothenbuhler A, Linglart A, Bougnères P. Higher methylation of the IGF1 P2 promoter is associated with idiopathic short stature. Clin Endocrinol (Oxf) 2016;84:216-21.
114. Chen J, Ying Y, Zhu H, et al. Curcumin-induced promoter hypermethylation of the mammalian target of rapamycin gene in multiple myeloma cells. Oncol Lett 2019;17:1108-14.
115. Rock CL. Milk and the risk and progression of cancer. Nestle Nutr Workshop Ser Pediatr Program 2011;67:173-85.
116. Manuelian CL, Penasa M, Visentin G, Zidi A, Cassandro M, De Marchi M. Mineral composition of cow milk from multibreed herds. Anim Sci J 2018;89:1622-7.
117. Chan JM, Stampfer MJ, Ma J, Gann PH, Gaziano JM, Giovannucci EL. Dairy products, calcium, and prostate cancer risk in the Physicians' Health Study. Am J Clin Nutr 2001;74:549-54.
118. Gao X, LaValley MP, Tucker KL. Prospective studies of dairy product and calcium intakes and prostate cancer risk: a meta-analysis. J Natl Cancer Inst 2005;97:1768-77.
119. Rodriguez C, McCullough ML, Mondul AM, et al. Calcium, dairy products, and risk of prostate cancer in a prospective cohort of United States men. Cancer Epidemiol Biomarkers Prev 2003;12:597-603.
120. Hayes RB, Ziegler RG, Gridley G, et al. Dietary factors and risks for prostate cancer among blacks and whites in the United States. Cancer Epidemiol Biomarkers Prev 1999;8:25-34.
121. Berndt SI, Carter H, Landis PK, et al. Calcium intake and prostate cancer risk in a long-term aging study: the Baltimore Longitudinal Study of Aging. Urology 2002;60:1118-23.
122. Aune D, Navarro Rosenblatt DA, Chan DS, et al. Dairy products, calcium, and prostate cancer risk: a systematic review and meta-analysis of cohort studies. Am J Clin Nutr 2015;101:87-117.
123. Maly IV, Hofmann WA. Fatty acids and calcium regulation in prostate cancer. Nutrients 2018;10:788.
124. Maly IV, Hofmann WA. Calcium and nuclear signaling in prostate cancer. Int J Mol Sci 2018;19:1237.
125. Harrison S, Lennon R, Holly J, et al. Does milk intake promote prostate cancer initiation or progression via effects on insulin-like growth factors (IGFs)? Cancer Causes Control 2017;28:497-528.
126. Mantzoros CS, Tzonou A, Signorello LB, Stampfer M, Trichopoulos D, Adami HO. Insulin-like growth factor 1 in relation to prostate cancer and benign prostatic hyperplasia. Br J Cancer 1997;76:1115-8.
127. Cohen P, Peehl DM, Rosenfeld R. Insulin-like growth factor 1 in relation to prostate cancer and benign prostatic hyperplasia. Br J Cancer 1998;78:554-6.
128. Wolk A, Mantzoros CS, Andersson SO, et al. Insulin-like growth factor 1 and prostate cancer risk: a population-based, case-control study. J Natl Cancer Inst 1998;90:911-5.
129. Kaplan PJ, Mohan S, Cohen P, Foster BA, Greenberg NM. The insulin-like growth factor axis and prostate cancer: lessons from the transgenic adenocarcinoma of mouse prostate (TRAMP) model. Cancer Res 1999;59:2203-9.
130. Giovannucci E. Insulin-like growth factor-I and binding protein-3 and risk of cancer. Horm Res 1999;51 Suppl 3:34-41.
131. Kaaks R, Lukanova A, Sommersberg B. Plasma androgens, IGF-1, body size, and prostate cancer risk: a synthetic review. Prostate Cancer Prostatic Dis 2000;3:157-72.
132. Chokkalingam AP, Pollak M, Fillmore CM, et al. Insulin-like growth factors and prostate cancer: a population-based case-control study in China. Cancer Epidemiol Biomarkers Prev 2001;10:421-7.
133. Nickerson T, Chang F, Lorimer D, Smeekens SP, Sawyers CL, Pollak M. In vivo progression of LAPC-9 and LNCaP prostate cancer models to androgen independence is associated with increased expression of insulin-like growth factor I (IGF-I) and IGF-I receptor (IGF-IR). Cancer Res 2001;61:6276-80.
134. Shi R, Berkel HJ, Yu H. Insulin-like growth factor-I and prostate cancer: a meta-analysis. Br J Cancer 2001;85:991-6.
135. Woodson K, Tangrea JA, Pollak M, et al. Serum insulin-like growth factor I: tumor marker or etiologic factor? Cancer Res 2003;63:3991-4.
136. Roberts CT. IGF-1 and prostate cancer. IGF-1 and prostate cancer. Novartis Found Symp 2004;262:193-9.
137. Renehan AG, Zwahlen M, Minder C, O'dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet 2004;363:1346-53.
138. Krueckl SL, Sikes RA, Edlund NM, et al. Increased insulin-like growth factor I receptor expression and signaling are components of androgen-independent progression in a lineage-derived prostate cancer progression model. Cancer Res 2004;64:8620-9.
139. Stattin P, Rinaldi S, Biessy C, Stenman UH, Hallmans G, Kaaks R. High levels of circulating insulin-like growth factor-I increase prostate cancer risk: a prospective study in a population-based nonscreened cohort. J Clin Oncol 2004;22:3104-12.
140. Platz EA, Pollak MN, Leitzmann MF, Stampfer MJ, Willett WC, Giovannucci E. Plasma insulin-like growth factor-1 and binding protein-3 and subsequent risk of prostate cancer in the PSA era. Cancer Causes Control 2005;16:255-62.
141. Gennigens C, Menetrier-Caux C, Droz JP. Insulin-like growth factor (IGF) family and prostate cancer. Crit Rev Oncol Hematol 2006;58:124-45.
142. Kawada M, Inoue H, Masuda T, Ikeda D. Insulin-like growth factor I secreted from prostate stromal cells mediates tumor-stromal cell interactions of prostate cancer. Cancer Res 2006;66:4419-25.
143. Wu JD, Haugk K, Woodke L, Nelson P, Coleman I, Plymate SR. Interaction of IGF signaling and the androgen receptor in prostate cancer progression. J Cell Biochem 2006;99:392-401.
144. Kawada M, Inoue H, Arakawa M, Ikeda D. Transforming growth factor-beta1 modulates tumor-stromal cell interactions of prostate cancer through insulin-like growth factor-I. Anticancer Res 2008;28:721-30.
145. Kojima S, Inahara M, Suzuki H, Ichikawa T, Furuya Y. Implications of insulin-like growth factor-I for prostate cancer therapies. Int J Urol 2009;16:161-7.
146. Nimptsch K, Platz EA, Pollak MN, et al. Plasma insulin-like growth factor 1 is positively associated with low-grade prostate cancer in the Health Professionals Follow-up Study 1993-2004. Int J Cancer 2011;128:660-7.
147. Heidegger I, Massoner P, Sampson N, Klocker H. The insulin-like growth factor (IGF) axis as an anticancer target in prostate cancer. Cancer Lett 2015;367:113-21.
148. Cao Y, Nimptsch K, Shui IM, et al. Prediagnostic plasma IGFBP-1, IGF-1 and risk of prostate cancer. Int J Cancer 2015;136:2418-26.
149. Ahearn TU, Peisch S, Pettersson A, et al. Transdisciplinary Prostate Cancer Partnership (ToPCaP). Expression of IGF/insulin receptor in prostate cancer tissue and progression to lethal disease. Carcinogenesis 2018;39:1431-7.
150. Kim M, Kim JW, Kim JK, et al. Association between serum levels of insulin-like growth factor-1, bioavailable testosterone, and pathologic Gleason score. Cancer Med 2018;7:4170-80.
151. Ohishi T, Abe H, Sakashita C, et al. Inhibition of mitochondria ATP synthase suppresses prostate cancer growth through reduced insulin-like growth factor-1 secretion by prostate stromal cells. Int J Cancer 2020;146:3474-84.
152. Melnik BC, John SM, Schmitz G. Milk is not just food but most likely a genetic transfection system activating mTORC1 signaling for postnatal growth. Nutr J 2013;12:103.
153. Melnik BC, Schmitz G. Milk's role as an epigenetic regulator in health and disease. Diseases 2017;5:12.
154. Vasconcelos A, Santos T, Ravasco P, Neves PM. Dairy products: is there an impact on promotion of prostate cancer? Front Nutr 2019;6:62.
155. Pereira PC. Milk nutritional composition and its role in human health. Nutrition 2014;30:619-27.
156. Tunick MH, Van Hekken DL. Dairy products and health: recent insights. J Agric Food Chem 2015;63:9381-8.
157. Thorning TK, Raben A, Tholstrup T, Soedamah-Muthu SS, Givens I, Astrup A. Milk and dairy products: good or bad for human health? Food Nutr Res 2016;60:32527.
158. Gil Á, Ortega RM. Introduction and executive summary of the supplement, role of milk and dairy products in health and prevention of noncommunicable chronic diseases: a series of systematic reviews. Adv Nutr 2019;10:S67-73.
159. Timon CM, O'Connor A, Bhargava N, Gibney ER, Feeney EL. Dairy consumption and metabolic health. Nutrients 2020;12:3040.
160. Melnik BC. Lifetime impact of Cow's milk on overactivation of mTORC1: from fetal to childhood overgrowth, acne, diabetes, cancers, and neurodegeneration. Biomolecules 2021;11:404.
162. Michaëlsson K, Wolk A, Langenskiöld S, et al. Milk intake and risk of mortality and fractures in women and men: cohort studies. BMJ 2014;349:g6015.
163. Tognon G, Nilsson LM, Shungin D, et al. Nonfermented milk and other dairy products: associations with all-cause mortality. Am J Clin Nutr 2017;105:1502-11.
164. Michaëlsson K, Wolk A, Melhus H, Byberg L. Milk, fruit and vegetable, and total antioxidant intakes in relation to mortality rates: cohort studies in women and men. Am J Epidemiol 2017;185:345-61.
165. Michaëlsson K, Byberg L. Mixing of apples and oranges in milk research: a cohort analysis of non-fermented milk intake and all-cause mortality. Nutrients 2020;12:1393.
166. Torniainen S, Hedelin M, Autio V, et al. Lactase persistence, dietary intake of milk, and the risk for prostate cancer in Sweden and Finland. Cancer Epidemiol Biomarkers Prev 2007;16:956-61.
167. Agarwal MM, Rana SV, Mandal AK, et al. Lactose intolerance in prostate cancer patients: incidence and associated factors. Scand J Gastroenterol 2008;43:270-6.
168. Torfadottir JE, Steingrimsdottir L, Mucci L, et al. Milk intake in early life and risk of advanced prostate cancer. Am J Epidemiol 2012;175:144-53.
169. Pettersson A, Kasperzyk JL, Kenfield SA, et al. Milk and dairy consumption among men with prostate cancer and risk of metastases and prostate cancer death. Cancer Epidemiol Biomarkers Prev 2012;21:428-36.
170. Song Y, Chavarro JE, Cao Y, et al. Whole milk intake is associated with prostate cancer-specific mortality among U.S. male physicians. J Nutr 2013;143:189-96.
171. Yang M, Kenfield SA, Van Blarigan EL, et al. Dairy intake after prostate cancer diagnosis in relation to disease-specific and total mortality. Int J Cancer 2015;137:2462-9.
172. Lu W, Chen H, Niu Y, Wu H, Xia D, Wu Y. Dairy products intake and cancer mortality risk: a meta-analysis of 11 population-based cohort studies. Nutr J 2016;15:91.
173. Downer MK, Batista JL, Mucci LA, et al. Dairy intake in relation to prostate cancer survival. Int J Cancer 2017;140:2060-9.
174. Steck SE, Omofuma OO, Su LJ, et al. Calcium, magnesium, and whole-milk intakes and high-aggressive prostate cancer in the North Carolina-Louisiana Prostate Cancer Project (PCaP). Am J Clin Nutr 2018;107:799-807.
175. Tat D, Kenfield SA, Cowan JE, et al. Milk and other dairy foods in relation to prostate cancer recurrence: data from the cancer of the prostate strategic urologic research endeavor (CaPSURE™). Prostate 2018;78:32-9.
176. Gaard M, Tretli S, Løken EB. Dietary fat and the risk of breast cancer: a prospective study of 25,892 Norwegian women. Int J Cancer 1995;63:13-7.
177. Ronco AL, De Stéfani E, Dáttoli R. Dairy foods and risk of breast cancer: a case-control study in Montevideo, Uruguay. Eur J Cancer Prev 2002;11:457-63.
178. Wang F, Yu L, Wang F, et al. Risk factors for breast cancer in women residing in urban and rural areas of eastern China. J Int Med Res 2015;43:774-89.
179. Galván-Salazar HR, Arreola-Cruz A, Madrigal-Pérez D, et al. Association of milk and meat consumption with the development of breast cancer in a western Mexican population. Breast Care (Basel) 2015;10:393-6.
180. Ji J, Sundquist J, Sundquist K. Lactose intolerance and risk of lung, breast and ovarian cancers: aetiological clues from a population-based study in Sweden. Br J Cancer 2015;112:149-52.
181. McCann SE, Hays J, Baumgart CW, Weiss EH, Yao S, Ambrosone CB. Usual consumption of specific dairy foods is associated with breast cancer in the Roswell Park cancer institute data bank and biorepository. Curr Dev Nutr 2017;1:e000422.
182. Fraser GE, Jaceldo-Siegl K, Orlich M, Mashchak A, Sirirat R, Knutsen S. Dairy, soy, and risk of breast cancer: those confounded milks. Int J Epidemiol 2020;49:1526-37.
183. Kaluza J, Komatsu S, Lauriola M, et al. Long-term consumption of non-fermented and fermented dairy products and risk of breast cancer by estrogen receptor status - Population-based prospective cohort study. Clin Nutr 2021;40:1966-73.
184. Duarte-Salles T, Fedirko V, Stepien M, et al. Dairy products and risk of hepatocellular carcinoma: the European Prospective Investigation into Cancer and Nutrition. Int J Cancer 2014;135:1662-72.
185. Yang W, Sui J, Ma Y, et al. A prospective study of dairy product intake and the risk of hepatocellular carcinoma in U.S. men and women. Int J Cancer 2020;146:1241-9.
186. Wang XJ, Jiang CQ, Zhang WS, et al. Milk consumption and risk of mortality from all-cause, cardiovascular disease and cancer in older people. Clin Nutr 2020;39:3442-51.
188. Wang J, Li X, Zhang D. Dairy product consumption and risk of non-hodgkin lymphoma: a meta-analysis. Nutrients 2016;8:120.
189. Guerrero-Zotano A, Mayer IA, Arteaga CL. PI3K/AKT/mTOR: role in breast cancer progression, drug resistance, and treatment. Cancer Metastasis Rev 2016;35:515-24.
190. Sharma VR, Gupta GK, Sharma AK, et al. PI3K/Akt/mTOR intracellular pathway and breast cancer: factors, mechanism and regulation. Curr Pharm Des 2017;23:1633-8.
191. Hare SH, Harvey AJ. mTOR function and therapeutic targeting in breast cancer. Am J Cancer Res 2017;7:383-404.
192. Liu J, Li HQ, Zhou FX, Yu JW, Sun L, Han ZH. Targeting the mTOR pathway in breast cancer. Tumour Biol 2017;39:1010428317710825.
193. Araki K, Miyoshi Y. Mechanism of resistance to endocrine therapy in breast cancer: the important role of PI3K/Akt/mTOR in estrogen receptor-positive, HER2-negative breast cancer. Breast Cancer 2018;25:392-401.
194. Butt G, Shahwar D, Qureshi MZ, et al. Role of mTORC1 and mTORC2 in breast cancer: therapeutic targeting of mTOR and its partners to overcome metastasis and drug resistance. Adv Exp Med Biol 2019;1152:283-92.
195. Sridharan S, Basu A. Distinct roles of mTOR targets S6K1 and S6K2 in breast cancer. Int J Mol Sci 2020;21:1199.
196. Xu BH, Li XX, Yang Y, et al. Aberrant amino acid signaling promotes growth and metastasis of hepatocellular carcinomas through Rab1A-dependent activation of mTORC1 by Rab1A. Oncotarget 2015;6:20813-28.
197. Ericksen RE, Lim SL, McDonnell E, et al. Loss of BCAA catabolism during carcinogenesis enhances mTORC1 activity and promotes tumor development and progression. Cell Metab 2019;29:1151-65.e6.
198. Ericksen RE, Han W. Malignant manipulaTORs of metabolism: suppressing BCAA catabolism to enhance mTORC1 activity. Mol Cell Oncol 2019;6:1585171.
199. Akula SM, Abrams SL, Steelman LS, et al. RAS/RAF/MEK/ERK, PI3K/PTEN/AKT/mTORC1 and TP53 pathways and regulatory miRs as therapeutic targets in hepatocellular carcinoma. Expert Opin Ther Targets 2019;23:915-29.
200. Ferrín G, Guerrero M, Amado V, Rodríguez-Perálvarez M, De la Mata M. Activation of mTOR signaling pathway in hepatocellular carcinoma. Int J Mol Sci 2020;21:1266.
201. Xu ZZ, Xia ZG, Wang AH, et al. Activation of the PI3K/AKT/mTOR pathway in diffuse large B cell lymphoma: clinical significance and inhibitory effect of rituximab. Ann Hematol 2013;92:1351-8.
202. Majchrzak A, Witkowska M, Smolewski P. Inhibition of the PI3K/Akt/mTOR signaling pathway in diffuse large B-cell lymphoma: current knowledge and clinical significance. Molecules 2014;19:14304-15.
203. Browne SH, Diaz-Perez JA, Preziosi M, et al. mTOR activity in AIDS-related diffuse large B-cell lymphoma. PLoS One 2017;12:e0170771.
204. Ricci JE, Chiche J. Metabolic reprogramming of non-Hodgkin's B-cell lymphomas and potential therapeutic strategies. Front Oncol 2018;8:556.
205. Howell JJ, Ricoult SJ, Ben-Sahra I, Manning BD. A growing role for mTOR in promoting anabolic metabolism. Biochem Soc Trans 2013;41:906-12.
206. Ben-Sahra I, Manning BD. mTORC1 signaling and the metabolic control of cell growth. Curr Opin Cell Biol 2017;45:72-82.
207. Rabanal-Ruiz Y, Korolchuk VI. mTORC1 and nutrient homeostasis: the central role of the lysosome. Int J Mol Sci 2018;19:818.
209. Condon KJ, Sabatini DM. Nutrient regulation of mTORC1 at a glance. J Cell Sci 2019;132:jcs222570.
210. Zhu M, Wang XQ. Regulation of mTORC1 by small GTPases in response to nutrients. J Nutr 2020;150:1004-11.
212. Rich-Edwards JW, Ganmaa D, Pollak MN, et al. Milk consumption and the prepubertal somatotropic axis. Nutr J 2007;6:28.
213. Barrea L, Di Somma C, Macchia PE, et al. Influence of nutrition on somatotropic axis: milk consumption in adult individuals with moderate-severe obesity. Clin Nutr 2017;36:293-301.
214. Grimberg A, Cohen P. Growth hormone and prostate cancer: guilty by association? J Endocrinol Invest 1999;22:64-73.
215. Weiss-Messer E, Merom O, Adi A, et al. Growth hormone (GH) receptors in prostate cancer: gene expression in human tissues and cell lines and characterization, GH signaling and androgen receptor regulation in LNCaP cells. Mol Cell Endocrinol 2004;220:109-23.
216. Bidosee M, Karry R, Weiss-Messer E, Barkey RJ. Regulation of growth hormone receptors in human prostate cancer cell lines. Mol Cell Endocrinol 2009;309:82-92.
217. Bidosee M, Karry R, Weiss-Messer E, Barkey RJ. Growth hormone affects gene expression and proliferation in human prostate cancer cells. Int J Androl 2011;34:124-37.
218. Nakonechnaya AO, Shewchuk BM. Growth hormone enhances LNCaP prostate cancer cell motility. Endocr Res 2015;40:97-105.
220. Lin S, Li C, Li C, Zhang X. Growth hormone receptor mutations related to individual dwarfism. Int J Mol Sci 2018;19:1433.
221. Shevah O, Laron Z. Patients with congenital deficiency of IGF-I seem protected from the development of malignancies: a preliminary report. Growth Horm IGF Res 2007;17:54-7.
222. Steuerman R, Shevah O, Laron Z. Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. Eur J Endocrinol 2011;164:485-9.
223. Lapkina-Gendler L, Rotem I, Pasmanik-Chor M, et al. Identification of signaling pathways associated with cancer protection in Laron syndrome. Endocr Relat Cancer 2016;23:399-410.
224. Werner H, Sarfstein R, Nagaraj K, Laron Z. Laron syndrome research paves the way for new insights in oncological investigation. Cells 2020;9:2446.
225. Wang Z, Prins GS, Coschigano KT, et al. Disruption of growth hormone signaling retards early stages of prostate carcinogenesis in the C3(1)/T antigen mouse. Endocrinology 2005;146:5188-96.
226. Wang Z, Luque RM, Kineman RD, et al. Disruption of growth hormone signaling retards prostate carcinogenesis in the Probasin/TAg rat. Endocrinology 2008;149:1366-76.
227. Muñoz-Moreno L, Schally AV, Prieto JC, Carmena MJ, Bajo AM. Growth hormone-releasing hormone receptor antagonists modify molecular machinery in the progression of prostate cancer. Prostate 2018;78:915-26.
228. Recouvreux MV, Wu JB, Gao AC, et al. Androgen receptor regulation of local growth hormone in prostate cancer cells. Endocrinology 2017;158:2255-68.
229. Rogers I, Emmett P, Gunnell D, Dunger D, Holly J. ALSPAC Study Tteam. Milk as a food for growth? Public Health Nutr 2006;9:359-68.
230. Norat T, Dossus L, Rinaldi S, et al. Diet, serum insulin-like growth factor-I and IGF-binding protein-3 in European women. Eur J Clin Nutr 2007;61:91-8.
231. Crowe FL, Key TJ, Allen NE, et al. The association between diet and serum concentrations of IGF-I, IGFBP-1, IGFBP-2, and IGFBP-3 in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev 2009;18:1333-40.
232. Qin LQ, He K, Xu JY. Milk consumption and circulating insulin-like growth factor-I level: a systematic literature review. Int J Food Sci Nutr 2009;60 Suppl 7:330-40.
233. Srinivasan V, Nimptsch K, Rohrmann S. Associations of current, childhood, and adolescent milk intake with serum insulin-like growth factor (IGF)-1 and IGF binding protein 3 concentrations in adulthood. Nutr Cancer 2019;71:931-8.
234. Romo Ventura E, Konigorski S, Rohrmann S, et al. Association of dietary intake of milk and dairy products with blood concentrations of insulin-like growth factor 1 (IGF-1) in Bavarian adults. Eur J Nutr 2020;59:1413-20.
235. Watling CZ, Kelly RK, Tong TYN, et al. Associations of circulating insulin-like growth factor-I with intake of dietary proteins and other macronutrients. Clin Nutr 2021;40:4685-93.
236. Hoppe C, Mølgaard C, Dalum C, Vaag A, Michaelsen KF. Differential effects of casein versus whey on fasting plasma levels of insulin, IGF-1 and IGF-1/IGFBP-3: results from a randomized 7-day supplementation study in prepubertal boys. Eur J Clin Nutr 2009;63:1076-83.
237. Blum JW, Baumrucker CR. Insulin-like growth factors (IGFs), IGF binding proteins, and other endocrine factors in milk: role in the newborn. Adv Exp Med Biol 2008;606:397-422.
238. Meyer Z, Höflich C, Wirthgen E, Olm S, Hammon HM, Hoeflich A. Analysis of the IGF-system in milk from farm animals - occurrence, regulation, and biomarker potential. Growth Horm IGF Res 2017;35:1-7.
239. Hoeflich A, Meyer Z. Functional analysis of the IGF-system in milk. Best Pract Res Clin Endocrinol Metab 2017;31:409-18.
240. Francis GL, Upton FM, Ballard FJ, McNeil KA, Wallace JC. Insulin-like growth factors 1 and 2 in bovine colostrum. Sequences and biological activities compared with those of a potent truncated form. Biochem J 1988;251:95-103.
241. Mauras N, Rogol AD, Haymond MW, Veldhuis JD. Sex steroids, growth hormone, insulin-like growth factor-1: neuroendocrine and metabolic regulation in puberty. Horm Res 1996;45:74-80.
242. Benyi E, Sävendahl L. The physiology of childhood growth: hormonal regulation. Horm Res Paediatr 2017;88:6-14.
243. Bromek E, Rysz M, Haduch A, Daniel WA. Serotonin receptors of 5-HT2 type in the hypothalamic arcuate nuclei positively regulate liver cytochrome P450 via stimulation of the growth hormone-releasing hormone/growth hormone hormonal pathway. Drug Metab Dispos 2019;47:80-5.
244. Vottero A, Guzzetti C, Loche S. New aspects of the physiology of the GH-IGF-1 axis. Endocr Dev 2013;24:96-105.
245. Takahashi Y. The role of growth hormone and insulin-like growth factor-i in the liver. Int J Mol Sci 2017;18:1447.
246. Harp JB, Goldstein S, Phillips LS. Nutrition and somatomedin. XXIII. Molecular regulation of IGF-I by amino acid availability in cultured hepatocytes. Diabetes 1991;40:95-101.
247. Wheelhouse NM, Stubbs AK, Lomax MA, MacRae JC, Hazlerigg DG. Growth hormone and amino acid supply interact synergistically to control insulin-like growth factor-I production and gene expression in cultured ovine hepatocytes. J Endocrinol 1999;163:353-61.
248. Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell 2005;4:119-25.
249. Dukes A, Davis C, El Refaey M, et al. The aromatic amino acid tryptophan stimulates skeletal muscle IGF1/p70s6k/mTor signaling in vivo and the expression of myogenic genes in vitro. Nutrition 2015;31:1018-24.
250. Fleddermann M, Demmelmair H, Grote V, et al. Role of selected amino acids on plasma IGF-I concentration in infants. Eur J Nutr 2017;56:613-20.
251. Oh HS, Oh SK, Lee JS, Wu C, Lee SJ. Effects of l-arginine on growth hormone and insulin-like growth factor 1. Food Sci Biotechnol 2017;26:1749-54.
252. Tsugawa Y, Handa H, Imai T. Arginine induces IGF-1 secretion from the endoplasmic reticulum. Biochem Biophys Res Commun 2019;514:1128-32.
253. Li B, Hock A, Wu RY, et al. Bovine milk-derived exosomes enhance goblet cell activity and prevent the development of experimental necrotizing enterocolitis. PLoS One 2019;14:e0211431.
254. Argon Y, Bresson SE, Marzec MT, Grimberg A. Glucose-regulated protein 94 (GRP94): a novel regulator of insulin-like growth factor production. Cells 2020;9:1844.
255. Ghiasi SM, Dahlby T, Hede Andersen C, et al. Endoplasmic reticulum chaperone glucose-regulated protein 94 is essential for proinsulin handling. Diabetes 2019;68:747-60.
256. Huang J, Manning BD. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J 2008;412:179-90.
257. Foster KG, Fingar DC. Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony. J Biol Chem 2010;285:14071-7.
258. Menon S, Dibble CC, Talbott G, et al. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell 2014;156:771-85.
259. Dibble CC, Cantley LC. Regulation of mTORC1 by PI3K signaling. Trends Cell Biol 2015;25:545-55.
260. Johnson SC. Nutrient sensing, signaling and ageing: the role of IGF-1 and mTOR in ageing and age-related disease. Subcell Biochem 2018;90:49-97.
261. Hoppe C, Mølgaard C, Michaelsen KF. Cow's milk and linear growth in industrialized and developing countries. Annu Rev Nutr 2006;26:131-73.
262. Mølgaard C, Larnkjær A, Arnberg K, Michaelsen KF. Milk and growth in children: effects of whey and casein. Nestle Nutr Workshop Ser Pediatr Program 2011;67:67-78.
263. Wiley AS. Cow milk consumption, insulin-like growth factor-I, and human biology: a life history approach. Am J Hum Biol 2012;24:130-8.
264. Martin RM, Holly JM, Gunnell D. Milk and linear growth: programming of the IGF-I axis and implication for health in adulthood. Nestle Nutr Workshop Ser Pediatr Program 2011;67:79-97.
265. Hyun S. Body size regulation and insulin-like growth factor signaling. Cell Mol Life Sci 2013;70:2351-65.
266. Ostman EM, Liljeberg Elmståhl HG, Björck IM. Inconsistency between glycemic and insulinemic responses to regular and fermented milk products. Am J Clin Nutr 2001;74:96-100.
267. Hoyt G, Hickey MS, Cordain L. Dissociation of the glycaemic and insulinaemic responses to whole and skimmed milk. Br J Nutr 2005;93:175-7.
268. Power O, Hallihan A, Jakeman P. Human insulinotropic response to oral ingestion of native and hydrolysed whey protein. Amino Acids 2009;37:333-9.
269. Yang J, Chi Y, Burkhardt BR, Guan Y, Wolf BA. Leucine metabolism in regulation of insulin secretion from pancreatic beta cells. Nutr Rev 2010;68:270-9.
270. Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrère B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci U S A 1997;94:14930-5.
271. Moore WT, Bowser SM, Fausnacht DW, Staley LL, Suh KS, Liu D. Beta cell function and the nutritional state: dietary factors that influence insulin secretion. Curr Diab Rep 2015;15:76.
272. Straus DS. Growth-stimulatory actions of insulin in vitro and in vivo. Endocr Rev 1984;5:356-69.
273. Sandow J. Growth effects of insulin and insulin analogues. Arch Physiol Biochem 2009;115:72-85.
274. Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 2011;12:21-35.
276. Tokarz VL, MacDonald PE, Klip A. The cell biology of systemic insulin function. J Cell Biol 2018;217:2273-89.
277. McGuire M, Beerman KA. Nutritional sciences: from fundamentals to food (with table of food composition booklet). 3rd ed. Boston, USA: Cengage Learning; 2018.
278. Lenders CM, Liu S, Wilmore DW, et al. Evaluation of a novel food composition database that includes glutamine and other amino acids derived from gene sequencing data. Eur J Clin Nutr 2009;63:1433-9.
279. Durán RV, Oppliger W, Robitaille AM, et al. Glutaminolysis activates Rag-mTORC1 signaling. Mol Cell 2012;47:349-58.
281. Avruch J, Long X, Ortiz-Vega S, Rapley J, Papageorgiou A, Dai N. Amino acid regulation of TOR complex 1. Am J Physiol Endocrinol Metab 2009;296:E592-602.
282. Kim SG, Buel GR, Blenis J. Nutrient regulation of the mTOR complex 1 signaling pathway. Mol Cells 2013;35:463-73.
283. Jewell JL, Russell RC, Guan KL. Amino acid signalling upstream of mTOR. Nat Rev Mol Cell Biol 2013;14:133-9.
285. Zheng X, Liang Y, He Q, et al. Current models of mammalian target of rapamycin complex 1 (mTORC1) activation by growth factors and amino acids. Int J Mol Sci 2014;15:20753-69.
286. Averous J, Lambert-Langlais S, Carraro V, et al. Requirement for lysosomal localization of mTOR for its activation differs between leucine and other amino acids. Cell Signal 2014;26:1918-27.
287. Oshiro N, Rapley J, Avruch J. Amino acids activate mammalian target of rapamycin (mTOR) complex 1 without changing Rag GTPase guanyl nucleotide charging. J Biol Chem 2014;289:2658-74.
288. Jewell JL, Kim YC, Russell RC, et al. Metabolism. Differential regulation of mTORC1 by leucine and glutamine. Science 2015;347:194-8.
289. Wang S, Tsun ZY, Wolfson RL, et al. Metabolism. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science 2015;347:188-94.
290. Duan Y, Li F, Tan K, et al. Key mediators of intracellular amino acids signaling to mTORC1 activation. Amino Acids 2015;47:857-67.
292. Powis K, De Virgilio C. Conserved regulators of Rag GTPases orchestrate amino acid-dependent TORC1 signaling. Cell Discov 2016;2:15049.
293. Nicastro R, Sardu A, Panchaud N, De Virgilio C. The architecture of the rag GTPase signaling network. Biomolecules 2017;7:48.
294. Wolfson RL, Sabatini DM. The dawn of the age of amino acid sensors for the mTORC1 pathway. Cell Metab 2017;26:301-9.
295. Ramlaul K, Aylett CHS. Signal integration in the (m)TORC1 growth pathway. Front Biol (Beijing) 2018;13:237-62.
296. Li XZ, Yan XH. Sensors for the mTORC1 pathway regulated by amino acids. J Zhejiang Univ Sci B 2019;20:699-712.
297. Zhuang Y, Wang XX, He J, He S, Yin Y. Recent advances in understanding of amino acid signaling to mTORC1 activation. Front Biosci (Landmark Ed) 2019;24:971-82.
298. Meng D, Yang Q, Wang H, et al. Glutamine and asparagine activate mTORC1 independently of Rag GTPases. J Biol Chem 2020;295:2890-9.
299. Segev N, Hay N. Hijacking leucyl-tRNA synthetase for amino acid-dependent regulation of TORC1. Mol Cell 2012;46:4-6.
300. Bonfils G, Jaquenoud M, Bontron S, Ostrowicz C, Ungermann C, De Virgilio C. Leucyl-tRNA synthetase controls TORC1 via the EGO complex. Mol Cell 2012;46:105-10.
301. Han JM, Jeong SJ, Park MC, et al. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell 2012;149:410-24.
302. Yoon MS, Son K, Arauz E, Han JM, Kim S, Chen J. Leucyl-tRNA synthetase activates Vps34 in amino acid-sensing mTORC1 signaling. Cell Rep 2016;16:1510-7.
303. Choi H, Son JB, Kang J, et al. Leucine-induced localization of Leucyl-tRNA synthetase in lysosome membrane. Biochem Biophys Res Commun 2017;493:1129-35.
304. Kim JH, Lee C, Lee M, et al. Control of leucine-dependent mTORC1 pathway through chemical intervention of leucyl-tRNA synthetase and RagD interaction. Nat Commun 2017;8:732.
305. Lee M, Kim JH, Yoon I, et al. Coordination of the leucine-sensing Rag GTPase cycle by leucyl-tRNA synthetase in the mTORC1 signaling pathway. Proc Natl Acad Sci U S A 2018;115:E5279-88.
306. Yoon I, Nam M, Kim HK, et al. Glucose-dependent control of leucine metabolism by leucyl-tRNA synthetase 1. Science 2020;367:205-10.
307. Carroll B, Maetzel D, Maddocks OD, et al. Control of TSC2-Rheb signaling axis by arginine regulates mTORC1 activity. Elife 2016;5:e11058.
308. Groenewoud MJ, Zwartkruis FJ. Rheb and Rags come together at the lysosome to activate mTORC1. Biochem Soc Trans 2013;41:951-5.
309. Jensen RG, Ferris AM, Lammi-keefe CJ, Henderson RA. Lipids of bovine and human milks: a comparison. J Dairy Sci 1990;73:223-40.
310. Qian L, Zhao A, Zhang Y, et al. Metabolomic approaches to explore chemical diversity of human breast-milk, formula milk and bovine milk. Int J Mol Sci 2016;17:2128.
311. Bourlieu C, Michalski MC. Structure-function relationship of the milk fat globule. Curr Opin Clin Nutr Metab Care 2015;18:118-27.
312. Bassingthwaighte JB, Noodleman L, van der Vusse G, Glatz JF. Modeling of palmitate transport in the heart. Mol Cell Biochem 1989;88:51-8.
313. Suiter C, Singha SK, Khalili R, Shariat-Madar Z. Free fatty acids: circulating contributors of metabolic syndrome. Cardiovasc Hematol Agents Med Chem 2018;16:20-34.
314. Shaw RJ. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol (Oxf) 2009;196:65-80.
315. Carroll B, Dunlop EA. The lysosome: a crucial hub for AMPK and mTORC1 signalling. Biochem J 2017;474:1453-66.
316. Hardie DG, Lin SC. AMP-activated protein kinase - not just an energy sensor. F1000Res 2017;6:1724.
317. Kwon B, Querfurth HW. Palmitate activates mTOR/p70S6K through AMPK inhibition and hypophosphorylation of raptor in skeletal muscle cells: reversal by oleate is similar to metformin. Biochimie 2015;118:141-50.
318. Yasuda M, Tanaka Y, Kume S, et al. Fatty acids are novel nutrient factors to regulate mTORC1 lysosomal localization and apoptosis in podocytes. Biochim Biophys Acta 2014;1842:1097-108.
319. Kumar S, Tikoo K. Independent role of PP2A and mTORc1 in palmitate induced podocyte death. Biochimie 2015;112:73-84.
320. Zhou YP, Wu R, Shen W, Yu HH, Yu SJ. Comparison of effects of oleic acid and palmitic acid on lipid deposition and mTOR/S6K1/SREBP-1c pathway in HepG2 cells. Zhonghua Gan Zang Bing Za Zhi 2018;26:451-6.
321. Tang NT, D Snook R, Brown MD, et al. Fatty-acid uptake in prostate cancer cells using dynamic microfluidic raman technology. Molecules 2020;25:1652.
322. Kurahashi N, Inoue M, Iwasaki M, Sasazuki S, Tsugane AS. Japan Public Health Center-Based Prospective Study Group. Dairy product, saturated fatty acid, and calcium intake and prostate cancer in a prospective cohort of Japanese men. Cancer Epidemiol Biomarkers Prev 2008;17:930-7.
323. Preble I, Zhang Z, Kopp R, et al. Dairy product consumption and prostate cancer risk in the United States. Nutrients 2019;11:1615.
324. Li H, Xu W, Ma Y, Zhou S, Xiao R. Milk fat globule membrane protein promotes C2C12 cell proliferation through the PI3K/Akt signaling pathway. Int J Biol Macromol 2018;114:1305-14.
325. Li H, Guan K, Li X, Ma Y, Zhou S. MFG-E8 induced differences in proteomic profiles in mouse C2C12 cells and its effect on PI3K/Akt and ERK signal pathways. Int J Biol Macromol 2019;124:681-8.
326. Jinushi M, Nakazaki Y, Carrasco DR, et al. Milk fat globule EGF-8 promotes melanoma progression through coordinated Akt and twist signaling in the tumor microenvironment. Cancer Res 2008;68:8889-98.
327. Soki FN, Koh AJ, Jones JD, et al. Polarization of prostate cancer-associated macrophages is induced by milk fat globule-EGF factor 8 (MFG-E8)-mediated efferocytosis. J Biol Chem 2014;289:24560-72.
328. Rikkert LG, de Rond L, van Dam A, et al. Detection of extracellular vesicles in plasma and urine of prostate cancer patients by flow cytometry and surface plasmon resonance imaging. PLoS One 2020;15:e0233443.
329. Reinhardt TA, Lippolis JD, Nonnecke BJ, Sacco RE. Bovine milk exosome proteome. J Proteomics 2012;75:1486-92.
330. Yamauchi M, Shimizu K, Rahman M, et al. Efficient method for isolation of exosomes from raw bovine milk. Drug Dev Ind Pharm 2019;45:359-64.
331. Rahman MM, Shimizu K, Yamauchi M, et al. Acidification effects on isolation of extracellular vesicles from bovine milk. PLoS One 2019;14:e0222613.
332. Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett 2016;371:48-61.
333. Khalifeh-Soltani A, McKleroy W, Sakuma S, et al. Mfge8 promotes obesity by mediating the uptake of dietary fats and serum fatty acids. Nat Med 2014;20:175-83.
334. Datta R, Lizama CO, Soltani AK, et al. Autoregulation of insulin receptor signaling through MFGE8 and the αvβ5 integrin. Proc Natl Acad Sci U S A 2021;118:e2102171118.
335. Heß K, Böger C, Behrens HM, Röcken C. Correlation between the expression of integrins in prostate cancer and clinical outcome in 1284 patients. Ann Diagn Pathol 2014;18:343-50.
336. Sun LC, Luo J, Mackey LV, Fuselier JA, Coy DH. A conjugate of camptothecin and a somatostatin analog against prostate cancer cell invasion via a possible signaling pathway involving PI3K/Akt, alphaVbeta3/alphaVbeta5 and MMP-2/-9. Cancer Lett 2007;246:157-66.
337. Welton JL, Brennan P, Gurney M, et al. Proteomics analysis of vesicles isolated from plasma and urine of prostate cancer patients using a multiplex, aptamer-based protein array. J Extracell Vesicles 2016;5:31209.
338. Lough AK. The phytanic acid content of the lipids of bovine tissues and milk. Lipids 1977;12:115-9.
339. Brown PJ, Mei G, Gibberd FB, et al. Diet and Refsum's disease. The determination of phytanic acid and phytol in certain foods and the application of this knowledge to the choice of suitable convenience foods for patients with Refsum's disease. J Hum Nutr Diet 1993;6:295-305.
340. Vetter W, Schröder M. Concentrations of phytanic acid and pristanic acid are higher in organic than in conventional dairy products from the German market. Food Chemistry 2010;119:746-52.
341. Roca-Saavedra P, Mariño-Lorenzo P, Miranda JM, et al. Phytanic acid consumption and human health, risks, benefits and future trends: a review. Food Chem 2017;221:237-47.
342. Wright ME, Bowen P, Virtamo J, Albanes D, Gann PH. Estimated phytanic acid intake and prostate cancer risk: a prospective cohort study. Int J Cancer 2012;131:1396-406.
343. Dhaunsi GS, Alsaeid M, Akhtar S. Phytanic acid attenuates insulin-like growth factor-1 activity via nitric oxide-mediated γ-secretase activation in rat aortic smooth muscle cells: possible implications for pathogenesis of infantile Refsum disease. Pediatr Res 2017;81:531-6.
344. Murakami D, Okamoto I, Nagano O, et al. Presenilin-dependent gamma-secretase activity mediates the intramembranous cleavage of CD44. Oncogene 2003;22:1511-6.
345. Okamoto I, Kawano Y, Tsuiki H, et al. CD44 cleavage induced by a membrane-associated metalloprotease plays a critical role in tumor cell migration. Oncogene 1999;18:1435-46.
346. Hao JL, Cozzi PJ, Khatri A, Power CA, Li Y. CD147/EMMPRIN and CD44 are potential therapeutic targets for metastatic prostate cancer. Curr Cancer Drug Targets 2010;10:287-306.
347. Liu C, Kelnar K, Liu B, et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med 2011;17:211-5.
348. Miletti-González KE, Murphy K, Kumaran MN, et al. Identification of function for CD44 intracytoplasmic domain (CD44-ICD): modulation of matrix metalloproteinase 9 (MMP-9) transcription via novel promoter response element. J Biol Chem 2012;287:18995-9007.
349. Xu H, Tian Y, Yuan X, et al. The role of CD44 in epithelial-mesenchymal transition and cancer development. Onco Targets Ther 2015;8:3783-92.
350. Lai CJ, Lin CY, Liao WY, Hour TC, Wang HD, Chuu CP. CD44 promotes migration and invasion of docetaxel-resistant prostate cancer cells likely via induction of hippo-yap signaling. Cells 2019;8:295.
351. Tsao T, Beretov J, Ni J, et al. Cancer stem cells in prostate cancer radioresistance. Cancer Lett 2019;465:94-104.
352. Okamoto I, Kawano Y, Murakami D, et al. Proteolytic release of CD44 intracellular domain and its role in the CD44 signaling pathway. J Cell Biol 2001;155:755-62.
353. Senbanjo LT, AlJohani H, Majumdar S, Chellaiah MA. Characterization of CD44 intracellular domain interaction with RUNX2 in PC3 human prostate cancer cells. Cell Commun Signal 2019;17:80.
354. van der Deen M, Akech J, Wang T, et al. The cancer-related Runx2 protein enhances cell growth and responses to androgen and TGFbeta in prostate cancer cells. J Cell Biochem 2010;109:828-37.
355. Fowler M, Borazanci E, McGhee L, et al. RUNX1 (AML-1) and RUNX2 (AML-3) cooperate with prostate-derived Ets factor to activate transcription from the PSA upstream regulatory region. J Cell Biochem 2006;97:1-17.
356. Little GH, Baniwal SK, Adisetiyo H, et al. Differential effects of RUNX2 on the androgen receptor in prostate cancer: synergistic stimulation of a gene set exemplified by SNAI2 and subsequent invasiveness. Cancer Res 2014;74:2857-68.
357. Schroeder TM, Jensen ED, Westendorf JJ. Runx2: a master organizer of gene transcription in developing and maturing osteoblasts. Birth Defects Res C Embryo Today 2005;75:213-25.
358. Baniwal SK, Khalid O, Gabet Y, et al. Runx2 transcriptome of prostate cancer cells: insights into invasiveness and bone metastasis. Mol Cancer 2010;9:258.
359. Baier SR, Nguyen C, Xie F, Wood JR, Zempleni J. MicroRNAs are absorbed in biologically meaningful amounts from nutritionally relevant doses of cow milk and affect gene expression in peripheral blood mononuclear cells, HEK-293 kidney cell cultures, and mouse livers. J Nutr 2014;144:1495-500.
360. Price AJ, Allen NE, Appleby PN, et al. Plasma phytanic acid concentration and risk of prostate cancer: results from the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr 2010;91:1769-76.
361. Wright ME, Albanes D, Moser AB, et al. Serum phytanic and pristanic acid levels and prostate cancer risk in Finnish smokers. Cancer Med 2014;3:1562-9.
362. Xu J, Thornburg T, Turner AR, et al. Serum levels of phytanic acid are associated with prostate cancer risk. Prostate 2005;63:209-14.
363. Thornburg T, Turner AR, Chen YQ, Vitolins M, Chang B, Xu J. Phytanic acid, AMACR and prostate cancer risk. Future Oncol 2006;2:213-23.
364. Hellgren LI. Phytanic acid--an overlooked bioactive fatty acid in dairy fat? Ann N Y Acad Sci 2010;1190:42-9.
365. Nóbrega M, Cilião HL, Souza MF, et al. Association of polymorphisms of PTEN, AKT1, PI3K, AR, and AMACR genes in patients with prostate cancer. Genet Mol Biol 2020;43:e20180329.
366. Kotova ES, Savochkina YA, Doludin YV, et al. Identification of clinically significant prostate cancer by combined PCA3 and AMACR mRNA detection in urine samples. Res Rep Urol 2020;12:403-13.
367. Lloyd MD, Darley DJ, Wierzbicki AS, Threadgill MD. Alpha-methylacyl-CoA racemase--an 'obscure' metabolic enzyme takes centre stage. FEBS J 2008;275:1089-102.
368. Kuefer R, Varambally S, Zhou M, et al. α-Methylacyl-CoA Racemase: expression levels of this novel cancer biomarker depend on tumor differentiation. Am J Pathol 2002;161:841-8.
369. Rubin MA, Bismar TA, Andrén O, et al. Decreased alpha-methylacyl CoA racemase expression in localized prostate cancer is associated with an increased rate of biochemical recurrence and cancer-specific death. Cancer Epidemiol Biomarkers Prev 2005;14:1424-32.
370. Barry M, Dhillon PK, Stampfer MJ, et al. α-Methylacyl-CoA racemase expression and lethal prostate cancer in the Physicians' Health Study and Health Professionals Follow-up Study. Prostate 2012;72:301-6.
371. Ananthanarayanan V, Deaton RJ, Yang XJ, Pins MR, Gann PH. Alpha-methylacyl-CoA racemase (AMACR) expression in normal prostatic glands and high-grade prostatic intraepithelial neoplasia (HGPIN): association with diagnosis of prostate cancer. Prostate 2005;63:341-6.
372. Zha S, Ferdinandusse S, Denis S, et al. Alpha-methylacyl-CoA racemase as an androgen-independent growth modifier in prostate cancer. Cancer Res 2003;63:7365-76.
373. Maruyama K, Oshima T, Ohyama K. Exposure to exogenous estrogen through intake of commercial milk produced from pregnant cows. Pediatr Int 2010;52:33-8.
374. Goyon A, Cai JZ, Kraehenbuehl K, Hartmann C, Shao B, Mottier P. Determination of steroid hormones in bovine milk by LC-MS/MS and their levels in Swiss Holstein cow milk. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2016;33:804-16.
375. Malekinejad H, Scherpenisse P, Bergwerff AA. Naturally occurring estrogens in processed milk and in raw milk (from gestated cows). J Agric Food Chem 2006;54:9785-91.
376. Tso J, Aga DS. A systematic investigation to optimize simultaneous extraction and liquid chromatography tandem mass spectrometry analysis of estrogens and their conjugated metabolites in milk. J Chromatogr A 2010;1217:4784-95.
377. Kolok AS, Ali JM, Rogan EG, Bartelt-Hunt SL. The fate of synthetic and endogenous hormones used in the US beef and dairy industries and the potential for human exposure. Curr Environ Health Rep 2018;5:225-32.
378. Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol 2013;63:800-9.
379. Bandini M, Gandaglia G, Briganti A. Obesity and prostate cancer. Curr Opin Urol 2017;27:415-21.
380. Schneider G, Kirschner MA, Berkowitz R, Ertel NH. Increased estrogen production in obese men. J Clin Endocrinol Metab 1979;48:633-8.
381. Carruba G. Estrogen and prostate cancer: an eclipsed truth in an androgen-dominated scenario. J Cell Biochem 2007;102:899-911.
382. Dobbs RW, Malhotra NR, Greenwald DT, Wang AY, Prins GS, Abern MR. Estrogens and prostate cancer. Prostate Cancer Prostatic Dis 2019;22:185-94.
383. Sehgal PD, Bauman TM, Nicholson TM, et al. Tissue-specific quantification and localization of androgen and estrogen receptors in prostate cancer. Hum Pathol 2019;89:99-108.
384. Tong da Y, Wen XQ, Jin Y, et al. Changes of androgen receptor and insulin-like growth factor-1 in LNCaP prostate cancer cells treated with sex hormones and flutamide. Asian Pac J Cancer Prev 2010;11:1805-9.
385. Tong da Y, Wu Xy, Sun Hy, Jin Y, Liu Zw, Zhou Fj. Expression changes and regulation of AR and IGF-1 in PC3 prostate cancer cells treated with sexual hormones and flutamide. Tumour Biol 2012;33:2151-8.
386. Bonkhoff H, Berges R. The evolving role of oestrogens and their receptors in the development and progression of prostate cancer. Eur Urol 2009;55:533-42.
387. Bonkhoff H. Estrogen receptor signaling in prostate cancer: implications for carcinogenesis and tumor progression. Prostate 2018;78:2-10.
388. Yu Z, Gao W, Jiang E, et al. Interaction between IGF-IR and ER induced by E2 and IGF-I. PLoS One 2013;8:e62642.
389. Lanzino M, Morelli C, Garofalo C, et al. Interaction between estrogen receptor alpha and insulin/IGF signaling in breast cancer. Curr Cancer Drug Targets 2008;8:597-610.
390. Hawsawi Y, El-Gendy R, Twelves C, Speirs V, Beattie J. Insulin-like growth factor - oestradiol crosstalk and mammary gland tumourigenesis. Biochim Biophys Acta 2013;1836:345-53.
391. Sun L, Gao Z, Luo L, Tan H, Zhang G. Estrogen affects cell growth and IGF-1 receptor expression in renal cell carcinoma. Onco Targets Ther 2018;11:5873-8.
392. Pandini G, Genua M, Frasca F, Vigneri R, Belfiore A. Sex steroids upregulate the IGF-1R in prostate cancer cells through a nongenotropic pathway. Ann N Y Acad Sci 2009;1155:263-7.
393. Alayev A, Salamon RS, Berger SM, et al. mTORC1 directly phosphorylates and activates ERα upon estrogen stimulation. Oncogene 2016;35:3535-43.
394. Migliaccio A, Castoria G, Di Domenico M, et al. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J 2000;19:5406-17.
395. Mannella P, Brinton RD. Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: a unified mechanism of estrogen action. J Neurosci 2006;26:9439-47.
396. Di Zazzo E, Galasso G, Giovannelli P, et al. Prostate cancer stem cells: the role of androgen and estrogen receptors. Oncotarget 2016;7:193-208.
397. Ohlsson JA, Johansson M, Hansson H, et al. Lactose, glucose and galactose content in milk, fermented milk and lactose-free milk products. Int Dairy J 2017;73:151-4.
398. Miles FL, Neuhouser ML, Zhang ZF. Concentrated sugars and incidence of prostate cancer in a prospective cohort. Br J Nutr 2018;120:703-10.
399. Marchesini G, Bua V, Brunori A, et al. Galactose elimination capacity and liver volume in aging man. Hepatology 1988;8:1079-83.
400. Schnegg M, Lauterburg BH. Quantitative liver function in the elderly assessed by galactose elimination capacity, aminopyrine demethylation and caffeine clearance. J Hepatol 1986;3:164-71.
401. Cui X, Zuo P, Zhang Q, et al. Chronic systemic D-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: protective effects of R-alpha-lipoic acid. J Neurosci Res 2006;84:647-54.
402. Sadigh-eteghad S, Majdi A, Mccann SK, et al. D-galactose-induced brain ageing model: a systematic review and meta-analysis on cognitive outcomes and oxidative stress indices. PLoS ONE 2017;12:e0184122.
403. Shwe T, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. Role of D-galactose-induced brain aging and its potential used for therapeutic interventions. Exp Gerontol 2018;101:13-36.
404. Azman KF, Zakaria R. D-Galactose-induced accelerated aging model: an overview. Biogerontology 2019;20:763-82.
405. Shukla S, Srivastava JK, Shankar E, et al. Oxidative stress and antioxidant status in high-risk prostate cancer subjects. Diagnostics (Basel) 2020;10:126.
406. Chen L, Yao H, Chen X, et al. Ginsenoside Rg1 decreases oxidative stress and down-regulates AKT/mTOR signalling to attenuate cognitive impairment in mice and senescence of neural stem cells induced by D-Galactose. Neurochem Res 2018;43:430-40.
407. Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res 2008;68:1777-85.
408. Khandrika L, Kumar B, Koul S, Maroni P, Koul HK. Oxidative stress in prostate cancer. Cancer Lett 2009;282:125-36.
409. Gupta-Elera G, Garrett AR, Robison RA, O'Neill KL. The role of oxidative stress in prostate cancer. Eur J Cancer Prev 2012;21:155-62.
410. Paschos A, Pandya R, Duivenvoorden WC, Pinthus JH. Oxidative stress in prostate cancer: changing research concepts towards a novel paradigm for prevention and therapeutics. Prostate Cancer Prostatic Dis 2013;16:217-25.
411. Udensi UK, Tchounwou PB. Oxidative stress in prostate hyperplasia and carcinogenesis. J Exp Clin Cancer Res 2016;35:139.
412. Kaya E, Ozgok Y, Zor M, et al. Oxidative stress parameters in patients with prostate cancer, benign prostatic hyperplasia and asymptomatic inflammatory prostatitis: a prospective controlled study. Adv Clin Exp Med 2017;26:1095-9.
413. Zhang Z, Jiang D, Wang C, et al. Polymorphisms in oxidative stress pathway genes and prostate cancer risk. Cancer Causes Control 2019;30:1365-75.
414. Ahmed Amar SA, Eryilmaz R, Demir H, Aykan S, Demir C. Determination of oxidative stress levels and some antioxidant enzyme activities in prostate cancer. Aging Male 2019;22:198-206.
415. Zhao Y, Hu X, Liu Y, et al. ROS signaling under metabolic stress: cross-talk between AMPK and AKT pathway. Mol Cancer 2017;16:79.
416. Bei Y, Wu X, Cretoiu D, et al. miR-21 suppression prevents cardiac alterations induced by d-galactose and doxorubicin. J Mol Cell Cardiol 2018;115:130-41.
417. Jiao G, Pan B, Zhou Z, Zhou L, Li Z, Zhang Z. MicroRNA-21 regulates cell proliferation and apoptosis in H2O2-stimulated rat spinal cord neurons. Mol Med Rep 2015;12:7011-6.
418. Golan-Gerstl R, Elbaum Shiff Y, Moshayoff V, Schecter D, Leshkowitz D, Reif S. Characterization and biological function of milk-derived miRNAs. Mol Nutr Food Res 2017;61:1700009.
419. Howard KM, Jati Kusuma R, Baier SR, et al. Loss of miRNAs during processing and storage of cow's (Bos taurus) milk. J Agric Food Chem 2015;63:588-92.
420. Manca S, Upadhyaya B, Mutai E, et al. Milk exosomes are bioavailable and distinct microRNA cargos have unique tissue distribution patterns. Sci Rep 2018;8:11321.
421. Kirchner B, Pfaffl MW, Dumpler J, von Mutius E, Ege MJ. microRNA in native and processed cow's milk and its implication for the farm milk effect on asthma. J Allergy Clin Immunol 2016;137:1893-1895.e13.
422. Wang L, Sadri M, Giraud D, Zempleni J. RNase H2-dependent polymerase chain reaction and elimination of confounders in sample collection, storage, and analysis strengthen evidence that microRNAs in Bovine milk are bioavailable in humans. J Nutr 2018;148:153-9.
423. Özdemir S. Identification and comparison of exosomal microRNAs in the milk and colostrum of two different cow breeds. Gene 2020;743:144609.
424. Kleinjan M, van Herwijnen MJ, Libregts SF, van Neerven RJ, Feitsma AL, Wauben MH. Regular industrial processing of Bovine milk impacts the integrity and molecular composition of extracellular vesicles. J Nutr 2021;151:1416-25.
425. Yu S, Zhao Z, Sun L, Li P. Fermentation results in quantitative changes in milk-derived exosomes and different effects on cell growth and survival. J Agric Food Chem 2017;65:1220-8.
426. Melnik BC, Schmitz G. Pasteurized non-fermented cow's milk but not fermented milk is a promoter of mTORC1-driven aging and increased mortality. Ageing Res Rev 2021;67:101270.
427. Izumi H, Kosaka N, Shimizu T, Sekine K, Ochiya T, Takase M. Bovine milk contains microRNA and messenger RNA that are stable under degradative conditions. J Dairy Sci 2012;95:4831-41.
428. Benmoussa A, Lee CH, Laffont B, et al. Commercial dairy Cow Milk microRNAs resist digestion under simulated gastrointestinal tract conditions. J Nutr 2016;146:2206-15.
429. Kusuma RJ, Manca S, Friemel T, Sukreet S, Nguyen C, Zempleni J. Human vascular endothelial cells transport foreign exosomes from cow's milk by endocytosis. Am J Physiol Cell Physiol 2016;310:C800-7.
430. Melnik BC, Kakulas F, Geddes DT, et al. Milk miRNAs: simple nutrients or systemic functional regulators? Nutr Metab (Lond) 2016;13:42.
431. Rani P, Vashisht M, Golla N, Shandilya S, Onteru SK, Singh D. Milk miRNAs encapsulated in exosomes are stable to human digestion and permeable to intestinal barrier in vitro. J Funct Foods 2017;34:431-9.
432. Lönnerdal B. Human milk microRNAs/Exosomes: composition and biological effects. Composition and biological effects. Nestlé Nutr Inst Workshop Ser 2019;90:83-92.
433. Lin D, Chen T, Xie M, et al. Oral administration of bovine and porcine milk exosome alter miRNAs profiles in piglet serum. Sci Rep 2020;10:6983.
434. Benmoussa A, Provost P. Milk MIcroRNAs in health and disease. Compr Rev Food Sci Food Saf 2019;18:703-22.
435. Carrillo-Lozano E, Sebastián-Valles F, Knott-Torcal C. Circulating microRNAs in breast milk and their potential impact on the infant. Nutrients 2020;12:3066.
436. Chen Z, Xie Y, Luo J, et al. Milk exosome-derived miRNAs from water buffalo are implicated in immune response and metabolism process. BMC Vet Res 2020;16:123.
437. Sadri M, Shu J, Kachman SD, Cui J, Zempleni J. Milk exosomes and miRNA cross the placenta and promote embryo survival in mice. Reproduction 2020;160:501-9.
438. Reif S, Elbaum Shiff Y, Golan-Gerstl R. Milk-derived exosomes (MDEs) have a different biological effect on normal fetal colon epithelial cells compared to colon tumor cells in a miRNA-dependent manner. J Transl Med 2019;17:325.
439. Ozkan H, Tuzun F, Taheri S, et al. Epigenetic programming through breast milk and its impact on milk-siblings mating. Front Genet 2020;11:569232.
440. Jin Y, Kotler JLM, Wang S, Huang B, Halpin JC, Street TO. The ER chaperones BiP and Grp94 regulate the formation of insulin-like growth factor 2 (IGF2) oligomers. J Mol Biol 2021;433:166963.
441. Lu T, Wang Y, Xu K, et al. Co-downregulation of GRP78 and GRP94 induces apoptosis and inhibits migration in prostate cancer cells. Open Life Sci 2019;14:384-91.
442. Le Doare K, Holder B, Bassett A, Pannaraj PS. Mother's milk: a purposeful contribution to the development of the infant microbiota and immunity. Front Immunol 2018;9:361.
443. Melnik BC, Schmitz G. MicroRNAs: milk's epigenetic regulators. Best Pract Res Clin Endocrinol Metab 2017;31:427-42.
444. Stremmel W, Weiskirchen R, Melnik BC. Milk exosomes prevent intestinal inflammation in a genetic mouse model of ulcerative colitis: a pilot experiment. Inflamm Intest Dis 2020;5:117-23.
445. van Esch BCAM, Porbahaie M, Abbring S, et al. The impact of milk and its components on epigenetic programming of immune function in early life and beyond: implications for allergy and asthma. Front Immunol 2020;11:2141.
446. Zempleni J, Aguilar-Lozano A, Sadri M, et al. Biological activities of extracellular vesicles and their cargos from bovine and human milk in humans and implications for infants. J Nutr 2017;147:3-10.
447. Zempleni J, Sukreet S, Zhou F, Wu D, Mutai E. Milk-derived exosomes and metabolic regulation. Annu Rev Anim Biosci 2019;7:245-62.
448. Xie MY, Hou LJ, Sun JJ, et al. Porcine milk exosome miRNAS attenuate LPS-induced apoptosis through inhibiting TLR4/NF-κB and p53 pathways in intestinal epithelial cells. J Agric Food Chem 2019;67:9477-91.
449. Melnik BC, Stremmel W, Weiskirchen R, John SM, Schmitz G. Exosome-derived microRNAs of human milk and their effects on infant health and development. Biomolecules 2021;11:851.
450. Melnik BC, Schmitz G. Exosomes of pasteurized milk: potential pathogens of Western diseases. J Transl Med 2019;17:3.
451. Chen X, Gao C, Li H, et al. Identification and characterization of microRNAs in raw milk during different periods of lactation, commercial fluid, and powdered milk products. Cell Res 2010;20:1128-37.
452. Fromm B, Tosar JP, Lu Y, Halushka MK, Witwer KW. Human and cow have identical miR-21-5p and miR-30a-5p sequences, which are likely unsuited to study dietary uptake from cow milk. J Nutr 2018;148:1506-7.
453. miRBase. hsa-mir-21-5p nucleotide sequence. Available from: http://mirbase.org/cgi-bin/mirna_entry.pl?acc=MIMAT0000076 [Last accessed on 17 Dec 2021].
454. miRBase. bta-mir-21-5p nucleotide sequence. Available from: http://mirbase.org/cgi-bin/mirna_entry.pl?acc=MIMAT0003528 [Last accessed on 17 Dec 2021].
455. Arntz OJ, Pieters BC, Oliveira MC, et al. Oral administration of bovine milk derived extracellular vesicles attenuates arthritis in two mouse models. Mol Nutr Food Res 2015;59:1701-12.
456. Marquez RT, Bandyopadhyay S, Wendlandt EB, et al. Correlation between microRNA expression levels and clinical parameters associated with chronic hepatitis C viral infection in humans. Lab Invest 2010;90:1727-36.
457. de Ceuninck van Capelle C, Spit M, Ten Dijke P. Current perspectives on inhibitory SMAD7 in health and disease. Crit Rev Biochem Mol Biol 2020;55:691-715.
458. Li L, Jiang D. Hypoxia-responsive miRNA-21-5p inhibits Runx2 suppression by targeting SMAD7 in MC3T3-E1 cells. J Cell Biochem 2019;120:16867-75.
460. Xiong Y, Tang Y, Fan F, et al. Exosomal hsa-miR-21-5p derived from growth hormone-secreting pituitary adenoma promotes abnormal bone formation in acromegaly. Transl Res 2020;215:1-16.
461. Edlund S, Lee SY, Grimsby S, et al. Interaction between Smad7 and beta-catenin: importance for transforming growth factor beta-induced apoptosis. Mol Cell Biol 2005;25:1475-88.
462. Shore P. A role for Runx2 in normal mammary gland and breast cancer bone metastasis. J Cell Biochem 2005;96:484-9.
463. Vishal M, Swetha R, Thejaswini G, Arumugam B, Selvamurugan N. Role of Runx2 in breast cancer-mediated bone metastasis. Int J Biol Macromol 2017;99:608-14.
464. Do DN, Li R, Dudemaine PL, Ibeagha-Awemu EM. MicroRNA roles in signalling during lactation: an insight from differential expression, time course and pathway analyses of deep sequence data. Sci Rep 2017;7:44605.
465. Benmoussa A, Ly S, Shan ST, et al. A subset of extracellular vesicles carries the bulk of microRNAs in commercial dairy cow's milk. J Extracell Vesicles 2017;6:1401897.
466. Benmoussa A, Laugier J, Beauparlant CJ, Lambert M, Droit A, Provost P. Complexity of the microRNA transcriptome of cow milk and milk-derived extracellular vesicles isolated via differential ultracentrifugation. J Dairy Sci 2020;103:16-29.
467. Le Guillou S, Leduc A, Laubier J, et al. Characterization of Holstein and Normande whole milk miRNomes highlights breed specificities. Sci Rep 2019;9:20345.
468. van Herwijnen MJC, Driedonks TAP, Snoek BL, et al. Abundantly present miRNAs in milk-derived extracellular vesicles are conserved between mammals. Front Nutr 2018;5:81.
469. Braud M, Magee DA, Park SD, et al. Genome-wide microRNA binding site variation between extinct wild aurochs and modern cattle identifies candidate microRNA-regulated domestication genes. Front Genet 2017;8:3.
470. Do DN, Dudemaine PL, Li R, Ibeagha-Awemu EM. Co-expression network and pathway analyses reveal important modules of miRNAs regulating milk yield and component traits. Int J Mol Sci 2017;18:1560.
471. miRBase. hsa-mir-148a-3p nucleotide sequence. Available from: http://mirbase.org/cgi-bin/mirna_entry.pl?acc=MIMAT0000243 [Last accessed on 17 Dec 2021].
472. miRBase. bta-mir-148a-3p nucleotide sequence. Available from: http://mirbase.org/cgi-bin/mirna_entry.pl?acc=MI0004737 [Last accessed on 17 Dec 2021].
473. Sanwlani R, Fonseka P, Chitti SV, Mathivanan S. Milk-derived extracellular vesicles in inter-organism, cross-species communication and drug delivery. Proteomes 2020;8:11.
474. Melnik BC, Kakulas F. Milk exosomes and microRNAs: potential epigenetic regulators. In: Patel V, Preedy V, editors. Handbook of nutrition, diet, and epigenetics. Cham: Springer International Publishing; 2017. p. 1-28.
475. Cao H, Wang L, Chen B, et al. DNA demethylation upregulated Nrf2 expression in Alzheimer's disease cellular model. Front Aging Neurosci 2015;7:244.
476. Bendavit G, Aboulkassim T, Hilmi K, Shah S, Batist G. Nrf2 transcription factor can directly regulate mTOR: linking cytoprotective gene expression to a major metabolic regulator that generates Redox activity. J Biol Chem 2016;291:25476-88.
477. Gao W, Ge S, Sun J. Ailanthone exerts anticancer effect by up-regulating miR-148a expression in MDA-MB-231 breast cancer cells and inhibiting proliferation, migration and invasion. Biomed Pharmacother 2019;109:1062-9.
478. TargetScanHuman, release 7.2. Available from: http://www.targetscan.org/cgi-bin/targetscan/vert_72/view_gene.cgi?rs=ENST00000287878.4&taxid=9606&members=miR-148-3p/152-3p&showcnc=0&shownc=0&shownc_nc=&showncf1=&showncf2=&subset=1 [Last accessed on 17 Dec 2021].
479. Gwinn DM, Shackelford DB, Egan DF, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 2008;30:214-26.
480. Oh S, Park MR, Son SJ, Kim Y. Comparison of total RNA isolation methods for analysis of immune-related microRNAs in market milks. Korean J Food Sci Anim Resour 2015;35:459-65.
481. Baddela VS, Nayan V, Rani P, Onteru SK, Singh D. Physicochemical biomolecular insights into buffalo milk-derived nanovesicles. Appl Biochem Biotechnol 2016;178:544-57.
482. El Tayebi HM, Waly AA, Assal RA, Hosny KA, Esmat G, Abdelaziz AI. Transcriptional activation of the IGF-II/IGF-1R axis and inhibition of IGFBP-3 by miR-155 in hepatocellular carcinoma. Oncol Lett 2015;10:3206-12.
483. Sun JF, Zhang D, Gao CJ, Zhang YW, Dai QS. Exosome-mediated MiR-155 transfer contributes to hepatocellular carcinoma cell proliferation by targeting PTEN. Med Sci Monit Basic Res 2019;25:218-28.
484. Tang B, Lei B, Qi G, et al. MicroRNA-155-3p promotes hepatocellular carcinoma formation by suppressing FBXW7 expression. J Exp Clin Cancer Res 2016;35:93.
485. Target ScanHuman. FBXW7 ENST00000281708.4. Available from: http://www.targetscan.org/cgibin/targetscan/vert_72/view_gene.cgi?rs=ENST00000281708.4&taxid=9606&members=&showcnc=0&shownc=0&showncf1=&showncf2=&subset=1 [Last accessed on 17 Dec 2021].
486. Mao JH, Kim IJ, Wu D, et al. FBXW7 targets mTOR for degradation and cooperates with PTEN in tumor suppression. Science 2008;321:1499-502.
487. Yeh CH, Bellon M, Nicot C. FBXW7: a critical tumor suppressor of human cancers. Mol Cancer 2018;17:115.
488. Lan R, Jin B, Liu YZ, Zhang K, Niu T, You Z. Genome and transcriptome profiling of FBXW family in human prostate cancer. Am J Clin Exp Urol 2020;8:116-28.
489. Beltran H, Rickman DS, Park K, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov 2011;1:487-95.
490. Li R, Dudemaine PL, Zhao X, Lei C, Ibeagha-Awemu EM. Comparative analysis of the miRNome of Bovine milk fat, whey and cells. PLoS One 2016;11:e0154129.
491. Ammah AA, Do DN, Bissonnette N, Gévry N, Ibeagha-Awemu EM. Co-expression network analysis identifies miRNA-mRNA networks potentially regulating milk traits and blood metabolites. Int J Mol Sci 2018;19:2500.
492. Le MT, Teh C, Shyh-Chang N, et al. MicroRNA-125b is a novel negative regulator of p53. Genes Dev 2009;23:862-76.
493. Kumar M, Lu Z, Takwi AA, et al. Negative regulation of the tumor suppressor p53 gene by microRNAs. Oncogene 2011;30:843-53.
494. Melnik BC. Milk disrupts p53 and DNMT1, the guardians of the genome: implications for acne vulgaris and prostate cancer. Nutr Metab (Lond) 2017;14:55.
495. Shi XB, Xue L, Ma AH, Tepper CG, Kung HJ, White RW. miR-125b promotes growth of prostate cancer xenograft tumor through targeting pro-apoptotic genes. Prostate 2011;71:538-49.
496. Amir S, Ma AH, Shi XB, Xue L, Kung HJ, Devere White RW. Oncomir miR-125b suppresses p14(ARF) to modulate p53-dependent and p53-independent apoptosis in prostate cancer. PLoS One 2013;8:e61064.
497. Downing SR, Russell PJ, Jackson P. Alterations of p53 are common in early stage prostate cancer. Can J Urol 2003;10:1924-33.
498. Feng Z. p53 regulation of the IGF-1/AKT/mTOR pathways and the endosomal compartment. Cold Spring Harb Perspect Biol 2010;2:a001057.
499. Buckbinder L, Talbott R, Velasco-Miguel S, et al. Induction of the growth inhibitor IGF-binding protein 3 by p53. Nature 1995;377:646-9.
500. Feng Z, Hu W, de Stanchina E, et al. The regulation of AMPK beta1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res 2007;67:3043-53.
501. Stambolic V, Macpherson D, Sas D, et al. Regulation of PTEN transcription by p53. Mol Cell 2001;8:317-25.
502. Feng Z, Zhang H, Levine AJ, Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci U S A 2005;102:8204-9.
503. Levine AJ, Feng Z, Mak TW, You H, Jin S. Coordination and communication between the p53 and IGF-1-AKT-TOR signal transduction pathways. Genes Dev 2006;20:267-75.
504. Alimirah F, Panchanathan R, Chen J, Zhang X, Ho SM, Choubey D. Expression of androgen receptor is negatively regulated by p53. Neoplasia 2007;9:1152-9.
505. Haupt S, Mejía-Hernández JO, Vijayakumaran R, Keam SP, Haupt Y. The long and the short of it: the MDM4 tail so far. J Mol Cell Biol 2019;11:231-44.
506. Stegeman S, Moya L, Selth LA, Spurdle AB, Clements JA, Batra J. A genetic variant of MDM4 influences regulation by multiple microRNAs in prostate cancer. Endocr Relat Cancer 2015;22:265-76.
507. Kotarac N, Dobrijevic Z, Matijasevic S, Savic-Pavicevic D, Brajuskovic G. Association of KLK3, VAMP8 and MDM4 genetic variants within microRNA binding sites with prostate cancer: evidence from Serbian population. Pathol Oncol Res 2020;26:2409-23.
508. Elmarakeby HA, Hwang J, Arafeh R, et al. Biologically informed deep neural network for prostate cancer discovery. Nature 2021;598:348-52.
509. Mecocci S, Pietrucci D, Milanesi M, et al. Transcriptomic characterization of cow, donkey and goat milk extracellular vesicles reveals their anti-inflammatory and immunomodulatory potential. Int J Mol Sci 2021;22:12759.
510. Correia NC, Gírio A, Antunes I, Martins LR, Barata JT. The multiple layers of non-genetic regulation of PTEN tumour suppressor activity. Eur J Cancer 2014;50:216-25.
511. Eguchi T, Watanabe K, Hara ES, Ono M, Kuboki T, Calderwood SK. OstemiR: a novel panel of microRNA biomarkers in osteoblastic and osteocytic differentiation from mesencymal stem cells. PLoS One 2013;8:e58796.
512. Wolf T, Baier SR, Zempleni J. The intestinal transport of bovine milk exosomes is mediated by endocytosis in human colon carcinoma Caco-2 cells and rat small intestinal IEC-6 cells. J Nutr 2015;145:2201-6.
513. Cicchini C, de Nonno V, Battistelli C, et al. Epigenetic control of EMT/MET dynamics: HNF4α impacts DNMT3s through miRs-29. Biochim Biophys Acta 2015;1849:919-29.
514. Bian Y, Lei Y, Wang C, et al. Epigenetic regulation of miR-29s affects the lactation activity of dairy cow mammary epithelial cells. J Cell Physiol 2015;230:2152-63.
515. Zhang J, Wang Y, Liu X, et al. Expression and potential role of microRNA-29b in mouse early embryo development. Cell Physiol Biochem 2015;35:1178-87.
516. Zhang Z, Cao Y, Zhai Y, et al. MicroRNA-29b regulates DNA methylation by targeting Dnmt3a/3b and Tet1/2/3 in porcine early embryo development. Dev Growth Differ 2018;60:197-204.
517. Mersey BD, Jin P, Danner DJ. Human microRNA (miR29b) expression controls the amount of branched chain alpha-ketoacid dehydrogenase complex in a cell. Hum Mol Genet 2005;14:3371-7.
518. Harris RA, Popov KM, Zhao Y, Shimomura Y. Regulation of branched-chain amino acid catabolism. J Nutr 1994;124:1499S-502S.
519. Doering CB, Williams IR, Danner DJ. Controlled overexpression of BCKD kinase expression: metabolic engineering applied to BCAA metabolism in a mammalian system. Metab Eng 2000;2:349-56.
520. Shimomura Y, Obayashi M, Murakami T, Harris RA. Regulation of branched-chain amino acid catabolism: nutritional and hormonal regulation of activity and expression of the branched-chain alpha-keto acid dehydrogenase kinase. Curr Opin Clin Nutr Metab Care 2001;4:419-23.
521. Nellis MM, Doering CB, Kasinski A, Danner DJ. Insulin increases branched-chain alpha-ketoacid dehydrogenase kinase expression in Clone 9 rat cells. Am J Physiol Endocrinol Metab 2002;283:E853-60.
522. Nie C, He T, Zhang W, Zhang G, Ma X. Branched chain amino acids: beyond nutrition metabolism. Int J Mol Sci 2018;19:954.
524. Worst TS, Previti C, Nitschke K, et al. miR-10a-5p and miR-29b-3p as extracellular vesicle-associated prostate cancer detection markers. Cancers (Basel) 2019;12:43.
525. Li Z, Hassan MQ, Jafferji M, et al. Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J Biol Chem 2009;284:15676-84.
526. Komori T. Regulation of proliferation, differentiation and functions of osteoblasts by Runx2. Int J Mol Sci 2019;20:1694.
528. Pratap J, Lian JB, Javed A, et al. Regulatory roles of Runx2 in metastatic tumor and cancer cell interactions with bone. Cancer Metastasis Rev 2006;25:589-600.
529. zur Hausen H, de Villiers EM. Dairy cattle serum and milk factors contributing to the risk of colon and breast cancers. Int J Cancer 2015;137:959-67.
530. Falida K, Eilebrecht S, Gunst K, Zur Hausen H, de Villiers EM. Isolation of two virus-like circular DNAs from commercially available milk samples. Genome Announc 2017;5:e00266-17.
531. zur Hausen H, Bund T, de Villiers E. Infectious agents in bovine red meat and milk and their potential role in cancer and other chronic diseases. Curr Top Microbiol Immunol 2017;407:83-116.
532. Eilebrecht S, Hotz-Wagenblatt A, Sarachaga V, et al. Expression and replication of virus-like circular DNA in human cells. Sci Rep 2018;8:2851.
533. Zur Hausen H, Bund T, de Villiers EM. Specific nutritional infections early in life as risk factors for human colon and breast cancers several decades later. Int J Cancer 2019;144:1574-83.
534. de Villiers EM, Gunst K, Chakraborty D, Ernst C, Bund T, Zur Hausen H. A specific class of infectious agents isolated from bovine serum and dairy products and peritumoral colon cancer tissue. Emerg Microbes Infect 2019;8:1205-18.
535. Bund T, Nikitina E, Chakraborty D, et al. Analysis of chronic inflammatory lesions of the colon for BMMF Rep antigen expression and CD68 macrophage interactions. Proc Natl Acad Sci U S A 2021;118:e2025830118.
536. de Villiers EM, Zur Hausen H. Bovine meat and milk factors (BMMFs): their proposed role in common human cancers and type 2 diabetes mellitus. Cancers (Basel) 2021;13:5407.
537. Prandini A, Tansini G, Sigolo S, Filippi L, Laporta M, Piva G. On the occurrence of aflatoxin M1 in milk and dairy products. Food Chem Toxicol 2009;47:984-91.
538. Ismail A, Akhtar S, Levin RE, Ismail T, Riaz M, Amir M. Aflatoxin M1: prevalence and decontamination strategies in milk and milk products. Crit Rev Microbiol 2016;42:418-27.
539. Hof H. Mycotoxins in milk for human nutrition: cow, sheep and human breast milk. GMS Infect Dis 2016;4:Doc03.
540. Marimón Sibaja KV, Gonçalves KDM, Garcia SO, et al. Aflatoxin M1 and B1 in Colombian milk powder and estimated risk exposure. Food Addit Contam Part B Surveill 2019;12:97-104.
541. Van der Fels-Klerx HJ, Vermeulen LC, Gavai AK, Liu C. Climate change impacts on aflatoxin B1 in maize and aflatoxin M1 in milk: a case study of maize grown in Eastern Europe and imported to the Netherlands. PLoS One 2019;14:e0218956.
542. Castegnaro M, Wild CP. IARC activities in mycotoxin research. Nat Toxins 1995;3:327-31; discussion 341.
543. IARC monographs on the identification of carcinogenic hazards to humans. Available from: https://monographs.iarc.fr/list-of-classifications (last updated 2020-03-03) [Last accessed on 17 Dec 2021].
544. Marchese S, Polo A, Ariano A, Velotto S, Costantini S, Severino L. Aflatoxin B1 and M1: biological properties and their involvement in cancer development. Toxins (Basel) 2018;10:214.
545. Vaz A, Cabral Silva AC, Rodrigues P, Venâncio A. Detection methods for aflatoxin M1 in dairy products. Microorganisms 2020;8:246.
546. Nguyen T, Flint S, Palmer J. Control of aflatoxin M1 in milk by novel methods: a review. Food Chem 2020;311:125984.
547. Scaglioni PT, Becker-Algeri T, Drunkler D, Badiale-Furlong E. Aflatoxin B1 and M1 in milk. Anal Chim Acta 2014;829:68-74.
548. Smith ER, Hagopian M. Uptake and secretion of carcinogenic chemicals by the dog and rat prostate. Prog Clin Biol Res 1981;75B:131-63.
549. Nishi N, Shoji H, Miyanaka H, Nakamura T. Androgen-regulated expression of a novel member of the aldo-keto reductase superfamily in regrowing rat prostate. Endocrinology 2000;141:3194-9.
550. Jin Y, Penning TM. Aldo-keto reductases and bioactivation/detoxication. Annu Rev Pharmacol Toxicol 2007;47:263-92.
551. Knight LP, Primiano T, Groopman JD, Kensler TW, Sutter TR. cDNA cloning, expression and activity of a second human aflatoxin B1-metabolizing member of the aldo-keto reductase superfamily, AKR7A3. Carcinogenesis 1999;20:1215-23.
552. Yepuru M, Wu Z, Kulkarni A, et al. Steroidogenic enzyme AKR1C3 is a novel androgen receptor-selective coactivator that promotes prostate cancer growth. Clin Cancer Res 2013;19:5613-25.
553. Karunasinghe N, Ambs S, Wang A, et al. Influence of lifestyle and genetic variants in the aldo-keto reductase 1C3 rs12529 polymorphism in high-risk prostate cancer detection variability assessed between US and New Zealand cohorts. PLoS One 2018;13:e0199122.
554. Karunasinghe N, Symes E, Gamage A, et al. Interaction between leukocyte aldo-keto reductase 1C3 activity, genotypes, biological, lifestyle and clinical features in a prostate cancer cohort from New Zealand. PLoS One 2019;14:e0217373.
555. Cui A, Hua H, Shao T, et al. Aflatoxin B1 induces Src phosphorylation and stimulates lung cancer cell migration. Tumour Biol 2015;36:6507-13.
556. Saad F, Lipton A. SRC kinase inhibition: targeting bone metastases and tumor growth in prostate and breast cancer. Cancer Treat Rev 2010;36:177-84.
557. Posadas EM, Ahmed RS, Karrison T, et al. Saracatinib as a metastasis inhibitor in metastatic castration-resistant prostate cancer: a University of Chicago Phase 2 Consortium and DOD/PCF Prostate Cancer Clinical Trials Consortium Study. Prostate 2016;76:286-93.
558. Li W, Wang Z, Wang L, et al. Effectiveness of inhibitor rapamycin, saracatinib, linsitinib and JNJ-38877605 against human prostate cancer cells. Int J Clin Exp Med 2015;8:6563-7.
559. Chakraborty G, Patail NK, Hirani R, et al. Attenuation of SRC kinase activity augments PARP inhibitor-mediated synthetic lethality in BRCA2-altered prostate tumors. Clin Cancer Res 2021;27:1792-806.
561. Marker PC, Donjacour AA, Dahiya R, Cunha GR. Hormonal, cellular, and molecular control of prostatic development. Dev Biol 2003;253:165-74.
562. Staack A, Donjacour AA, Brody J, Cunha GR, Carroll P. Mouse urogenital development: a practical approach. Differentiation 2003;71:402-13.
563. Thomson AA, Marker PC. Branching morphogenesis in the prostate gland and seminal vesicles. Differentiation 2006;74:382-92.
564. Ruan W, Powell-Braxton L, Kopchick JJ, Kleinberg DL. Evidence that insulin-like growth factor I and growth hormone are required for prostate gland development. Endocrinology 1999;140:1984-9.
565. Kleinberg DL, Ruan W, Yee D, Kovacs KT, Vidal S. Insulin-like growth factor (IGF)-I controls prostate fibromuscular development: IGF-I inhibition prevents both fibromuscular and glandular development in eugonadal mice. Endocrinology 2007;148:1080-8.
566. Ohlson N, Bergh A, Persson ML, Wikström P. Castration rapidly decreases local insulin-like growth factor-1 levels and inhibits its effects in the ventral prostate in mice. Prostate 2006;66:1687-97.
567. Ohlsson C, Mohan S, Sjögren K, et al. The role of liver-derived insulin-like growth factor-I. Endocr Rev 2009;30:494-535.
568. Banjac L, Kotur-Stevuljević J, Gojković T, Bokan-Mirković V, Banjac G, Banjac G. Relationship between insulin-like growth factor type 1 and intrauterine growth. Acta Clin Croat 2020;59:91-6.
569. Singal SS, Nygard K, Gratton R, Jansson T, Gupta MB. Increased insulin-like growth factor binding protein-1 phosphorylation in decidualized stromal mesenchymal cells in human intrauterine growth restriction placentas. J Histochem Cytochem 2018;66:617-30.
570. Agrogiannis GD, Sifakis S, Patsouris ES, Konstantinidou AE. Insulin-like growth factors in embryonic and fetal growth and skeletal development (Review). Mol Med Rep 2014;10:579-84.
571. Ghosh S, Lau H, Simons BW, et al. PI3K/mTOR signaling regulates prostatic branching morphogenesis. Dev Biol 2011;360:329-42.
572. Li Y, Ge C, Franceschi RT. Role of Runx2 in prostate development and stem cell function. Prostate 2021;81:231-41.
573. Voerman E, Gaillard R, Geurtsen ML, Jaddoe VWV. Maternal first-trimester cow-milk intake is positively associated with childhood general and abdominal visceral fat mass and lean mass but not with other cardiometabolic risk factors at the age of 10 Years. J Nutr 2021;151:1965-75.
574. Roland MC, Friis CM, Voldner N, et al. Fetal growth versus birthweight: the role of placenta versus other determinants. PLoS One 2012;7:e39324.
575. Tibblin G, Eriksson M, Cnattingius S, Ekbom A. High birthweight as a predictor of prostate cancer risk. Epidemiology 1995;6:423-4.
576. Eriksson M, Wedel H, Wallander MA, et al. The impact of birth weight on prostate cancer incidence and mortality in a population-based study of men born in 1913 and followed up from 50 to 85 years of age. Prostate 2007;67:1247-54.
577. Zhou CK, Sutcliffe S, Welsh J, et al. Is birthweight associated with total and aggressive/lethal prostate cancer risks? Br J Cancer 2016;114:839-48.
578. Cnattingius S, Lundberg F, Sandin S, Grönberg H, Iliadou A. Birth characteristics and risk of prostate cancer: the contribution of genetic factors. Cancer Epidemiol Biomarkers Prev 2009;18:2422-6.
579. Lahmann PH, Wallström P, Lissner L, Olsson H, Gullberg B. Measures of birth size in relation to risk of prostate cancer: the Malmö Diet and Cancer Study, Sweden. J Dev Orig Health Dis 2012;3:442-9.
580. Heppe DH, van Dam RM, Willemsen SP, et al. Maternal milk consumption, fetal growth, and the risks of neonatal complications: the Generation R Study. Am J Clin Nutr 2011;94:501-9.
581. Olsen SF, Halldorsson TI, Willett WC, et al. NUTRIX Consortium. Milk consumption during pregnancy is associated with increased infant size at birth: prospective cohort study. Am J Clin Nutr 2007;86:1104-10.
582. Brantsæter AL, Olafsdottir AS, Forsum E, Olsen SF, Thorsdottir I. Does milk and dairy consumption during pregnancy influence fetal growth and infant birthweight? Food Nutr Res 2012;56:20050.
583. Melnik BC, John SM, Schmitz G. Milk consumption during pregnancy increases birth weight, a risk factor for the development of diseases of civilization. J Transl Med 2015;13:13.
584. Achón M, Úbeda N, García-González Á, Partearroyo T, Varela-Moreiras G. Effects of milk and dairy product consumption on pregnancy and lactation outcomes: a systematic review. Adv Nutr 2019;10:S74-87.
585. Jiang H, Wu W, Zhang M, et al. Aberrant upregulation of miR-21 in placental tissues of macrosomia. J Perinatol 2014;34:658-63.
586. Zhang JT, Cai QY, Ji SS, et al. Decreased miR-143 and increased miR-21 placental expression levels are associated with macrosomia. Mol Med Rep 2016;13:3273-80.
587. Wen HY, Abbasi S, Kellems RE, Xia Y. mTOR: a placental growth signaling sensor. Placenta 2005;26 Suppl A:S63-9.
588. Roos S, Lagerlöf O, Wennergren M, Powell TL, Jansson T. Regulation of amino acid transporters by glucose and growth factors in cultured primary human trophoblast cells is mediated by mTOR signaling. Am J Physiol Cell Physiol 2009;297:C723-31.
589. Jansson T, Aye IL, Goberdhan DC. The emerging role of mTORC1 signaling in placental nutrient-sensing. Placenta 2012;33 Suppl 2:e23-9.
590. Rosario FJ, Kanai Y, Powell TL, Jansson T. Mammalian target of rapamycin signalling modulates amino acid uptake by regulating transporter cell surface abundance in primary human trophoblast cells. J Physiol 2013;591:609-25.
591. Xu J, Lu C, Wang J, Zhang R, Qian X, Zhu H. Regulation of human trophoblast GLUT3 glucose transporter by mammalian target of rapamycin signaling. Int J Mol Sci 2015;16:13815-28.
592. Rosario FJ, Powell TL, Gupta MB, Cox L, Jansson T. mTORC1 transcriptional regulation of ribosome subunits, protein synthesis, and molecular transport in primary human trophoblast cells. Front Cell Dev Biol 2020;8:583801.
593. Cook MB, Gamborg M, Aarestrup J, Sørensen TI, Baker JL. Childhood height and birth weight in relation to future prostate cancer risk: a cohort study based on the copenhagen school health records register. Cancer Epidemiol Biomarkers Prev 2013;22:2232-40.
594. Aarestrup J, Gamborg M, Cook MB, Baker JL. Childhood height increases the risk of prostate cancer mortality. Eur J Cancer 2015;51:1340-5.
595. Aarestrup J, Bjerregaard LG, Meyle KD, et al. Birthweight, childhood overweight, height and growth and adult cancer risks: a review of studies using the Copenhagen School Health Records Register. Int J Obes (Lond) 2020;44:1546-60.
596. Bjerregaard LG, Aarestrup J, Gamborg M, Lange T, Tjønneland A, Baker JL. Childhood height, adult height, and the risk of prostate cancer. Cancer Causes Control 2016;27:561-7.
597. Alimujiang A, Colditz GA, Gardner JD, Park Y, Berkey CS, Sutcliffe S. Childhood diet and growth in boys in relation to timing of puberty and adult height: the Longitudinal Studies of Child Health and Development. Cancer Causes Control 2018;29:915-26.
598. Wiley AS. Consumption of milk, but not other dairy products, is associated with height among US preschool children in NHANES 1999-2002. Ann Hum Biol 2009;36:125-38.
600. Almon R, Nilsson TK, Sjöström M, Engfeldt P. Lactase persistence and milk consumption are associated with body height in Swedish preadolescents and adolescents. Food Nutr Res 2011;55:7253.
601. Melnik BC. Evidence for acne-promoting effects of milk and other insulinotropic dairy products. Nestle Nutr Workshop Ser Pediatr Program 2011;67:131-45.
602. Adebamowo CA, Spiegelman D, Danby FW, Frazier AL, Willett WC, Holmes MD. High school dietary dairy intake and teenage acne. J Am Acad Dermatol 2005;52:207-14.
603. Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in adolescent girls. Dermatol Online J 2006:12.
604. Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in teenaged boys. J Am Acad Dermatol 2008;58:787-93.
605. Juhl CR, Bergholdt HKM, Miller IM, Jemec GBE, Kanters JK, Ellervik C. Dairy intake and acne vulgaris: a systematic review and meta-analysis of 78,529 children, adolescents, and young adults. Nutrients 2018;10:1049.
606. Aghasi M, Golzarand M, Shab-Bidar S, Aminianfar A, Omidian M, Taheri F. Dairy intake and acne development: a meta-analysis of observational studies. Clin Nutr 2019;38:1067-75.
607. Dai R, Hua W, Chen W, Xiong L, Li L. The effect of milk consumption on acne: a meta-analysis of observational studies. J Eur Acad Dermatol Venereol 2018;32:2244-53.
608. Robeva R, Assyov Y, Tomova A, Kumanov P. Acne vulgaris is associated with intensive pubertal development and altitude of residence-a cross-sectional population-based study on 6,200 boys. Eur J Pediatr 2013;172:465-71.
609. Melnik BC, Zouboulis CC. Potential role of FoxO1 and mTORC1 in the pathogenesis of Western diet-induced acne. Exp Dermatol 2013;22:311-5.
610. Monfrecola G, Lembo S, Caiazzo G, et al. Mechanistic target of rapamycin (mTOR) expression is increased in acne patients' skin. Exp Dermatol 2016;25:153-5.
611. Agamia NF, Abdallah DM, Sorour O, Mourad B, Younan DN. Skin expression of mammalian target of rapamycin and forkhead box transcription factor O1, and serum insulin-like growth factor-1 in patients with acne vulgaris and their relationship with diet. Br J Dermatol 2016;174:1299-307.
612. Galobardes B, Davey Smith G, Jeffreys M, Kinra S, McCarron P. Acne in adolescence and cause-specific mortality: lower coronary heart disease but higher prostate cancer mortality: the Glasgow Alumni Cohort Study. Am J Epidemiol 2005;161:1094-101.
613. Sutcliffe S, Giovannucci E, Isaacs WB, Willett WC, Platz EA. Acne and risk of prostate cancer. Int J Cancer 2007;121:2688-92.
614. Ugge H, Udumyan R, Carlsson J, et al. Acne in late adolescence and risk of prostate cancer. Int J Cancer 2018;142:1580-5.
615. Tate PL, Bibb R, Larcom LL. Milk stimulates growth of prostate cancer cells in culture. Nutr Cancer 2011;63:1361-6.
616. Park SW, Kim JY, Kim YS, Lee SJ, Lee SD, Chung MK. A milk protein, casein, as a proliferation promoting factor in prostate cancer cells. World J Mens Health 2014;32:76-82.
617. Gordon WG, Semmett WF, Cable RS, Morris M. Amino acid composition of α-casein and β-casein2. J Am Chem Soc 1949;71:3293-7.
618. Kim JY, Bang SI, Lee SD. α-casein changes gene expression profiles and promotes tumorigenesis of prostate cancer cells. Nutr Cancer 2020;72:239-51.
619. Bernichtein S, Pigat N, Capiod T, et al. High milk consumption does not affect prostate tumor progression in two mouse models of benign and neoplastic lesions. PLoS One 2015;10:e0125423.
620. Larsson SC, Mason AM, Kar S, et al. Genetically proxied milk consumption and risk of colorectal, bladder, breast, and prostate cancer: a two-sample Mendelian randomization study. BMC Med 2020;18:370.
621. Clausnitzer J. Statista. Per capita consumption of milk in Finland 2010-2020. Available from: https://www.statista.com/statistics/460031/per-capita-consumption-of-milk-in-finland/ [Last accessed on 17 Dec 2021].
622. Vissers LET, Sluijs I, van der Schouw YT, et al. Dairy product intake and risk of type 2 diabetes in EPIC-interAct: a mendelian randomization study. Diabetes Care 2019;42:568-75.
623. Kitsiou-Tzeli S, Tzetis M. Maternal epigenetics and fetal and neonatal growth. Curr Opin Endocrinol Diabetes Obes 2017;24:43-6.
624. Cullen SM, Hassan N, Smith-Raska M. Effects of noninherited ancestral genotypes on offspring phenotypes†. Biol Reprod 2021;105:747-60.
625. Godos Godos J, Tieri M, Ghelfi F, et al. Dairy foods and health: an umbrella review of observational studies. Int J Food Sci Nutr 2020;71:138-51.
626. Cavero-Redondo I, Alvarez-Bueno C, Sotos-Prieto M, Gil A, Martinez-Vizcaino V, Ruiz JR. Milk and dairy product consumption and risk of mortality: an overview of systematic reviews and meta-analyses. Adv Nutr 2019;10:S97-S104.
627. López-Plaza B, Bermejo LM, Santurino C, Cavero-Redondo I, Álvarez-Bueno C, Gómez-Candela C. Milk and dairy product consumption and prostate cancer risk and mortality: an overview of systematic reviews and meta-analyses. Adv Nutr 2019;10:S212-23.
628. Ong SL, Blenkiron C, Haines S, et al. Ruminant milk-derived extracellular vesicles: a nutritional and therapeutic opportunity? Nutrients 2021;13:2505.
629. Zhang Y, Xu Q, Hou J, et al. Loss of bioactive microRNAs in cow's milk by ultra-high-temperature treatment but not by pasteurization treatment. J Sci Food Agric 2021; doi: 10.1002/jsfa.11607.
630. The American College of Obstreticians and Gynecologists. Nutrition during pregnancy. Available from: https://www.acog.org/womens-health/faqs/nutrition-during-pregnancy [Last accessed on 17 Dec 2021].
631. John Hopkins Medicine. Eating safely during pregnancy. Available from: https://www.hopkinsmedicine.org/health/wellness-and-prevention/-/media/ksw-images/eatingsafely [Last accessed on 17 Dec 2021].
633. Government UK. Guidance. Eligibility for the school milk subsidy scheme - milk consumed from 1 January 2021. Available from: https://www.gov.uk/guidance/eligibility-for-the-school-milk-subsidy-scheme-milk-consumed-from-1-january-2021 [Last accessed on 17 Dec 2021].
634. Agostoni C, Turck D. Is cow's milk harmful to a child's health? J Pediatr Gastroenterol Nutr 2011;53:594-600.
635. Everett S, Joshi R, Galmer L, Goolsby M, Lane J. Diet and nutrition in orthopedics. In: Rajendram R, Preedy VR, Patel VB, editors. Diet and nutrition in critical care. New York: Springer; 2014. p. 1-20.
636. Fardellone P, Séjourné A, Blain H, Cortet B, Thomas T. GRIO Scientific Committee. Osteoporosis: is milk a kindness or a curse? Joint Bone Spine 2017;84:275-81.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Melnik BC, John SM, Weiskirchen R, Schmitz G. The endocrine and epigenetic impact of persistent cow milk consumption on prostate carcinogenesis. J Transl Genet Genom 2022;6:1-45. http://dx.doi.org/10.20517/jtgg.2021.37
AMA Style
Melnik BC, John SM, Weiskirchen R, Schmitz G. The endocrine and epigenetic impact of persistent cow milk consumption on prostate carcinogenesis. Journal of Translational Genetics and Genomics. 2022; 6(1): 1-45. http://dx.doi.org/10.20517/jtgg.2021.37
Chicago/Turabian Style
Melnik, Bodo C., Swen Malte John, Ralf Weiskirchen, Gerd Schmitz. 2022. "The endocrine and epigenetic impact of persistent cow milk consumption on prostate carcinogenesis" Journal of Translational Genetics and Genomics. 6, no.1: 1-45. http://dx.doi.org/10.20517/jtgg.2021.37
ACS Style
Melnik, BC.; John SM.; Weiskirchen R.; Schmitz G. The endocrine and epigenetic impact of persistent cow milk consumption on prostate carcinogenesis. J. Transl. Genet. Genom. 2022, 6, 1-45. http://dx.doi.org/10.20517/jtgg.2021.37
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 21 clicks
Cite This Article 21 clicks


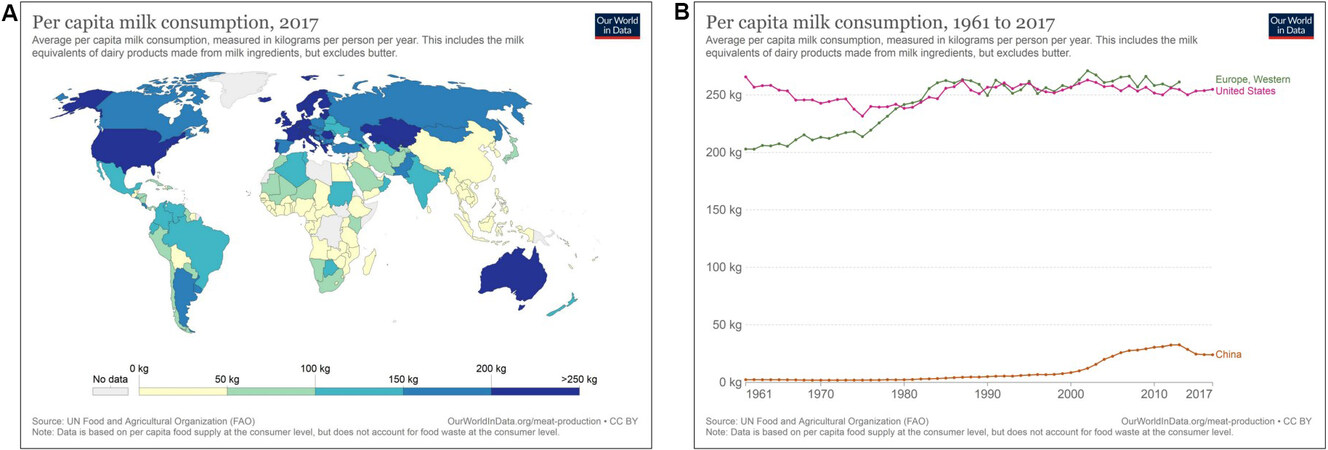
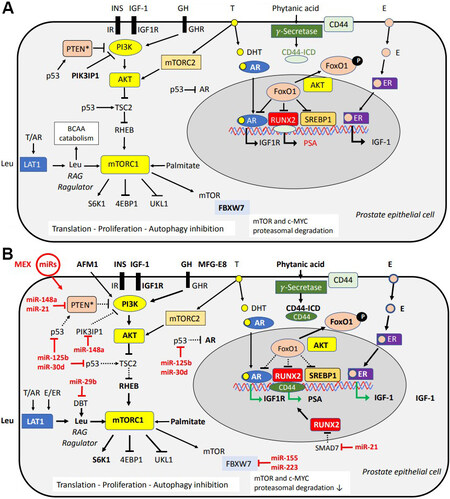
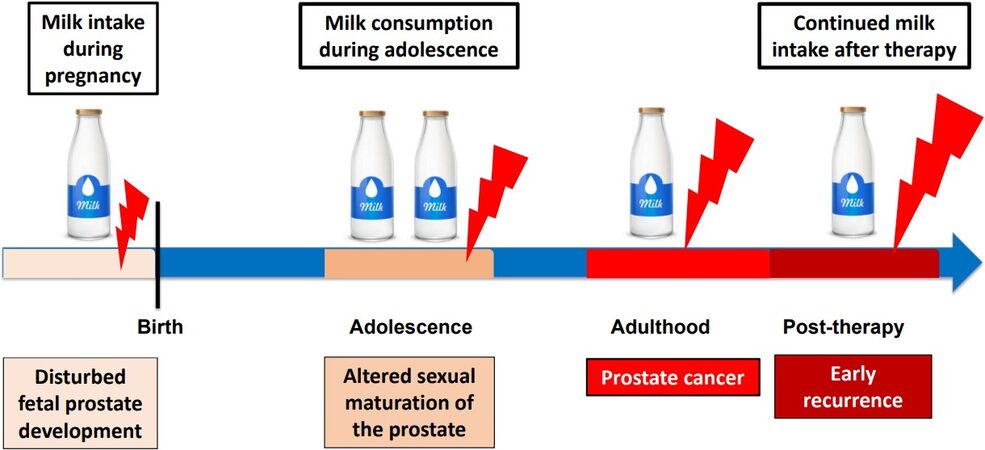
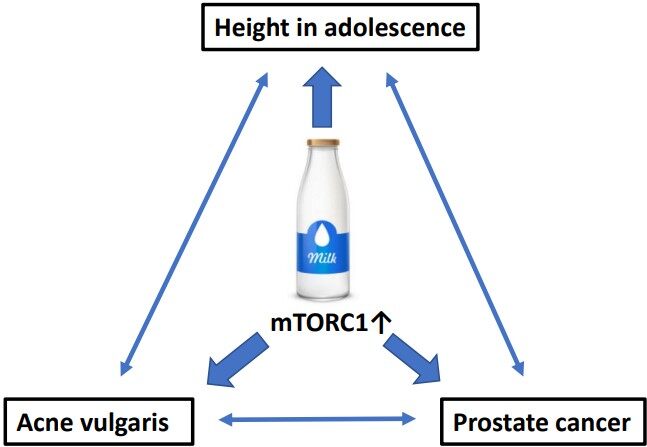









Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.