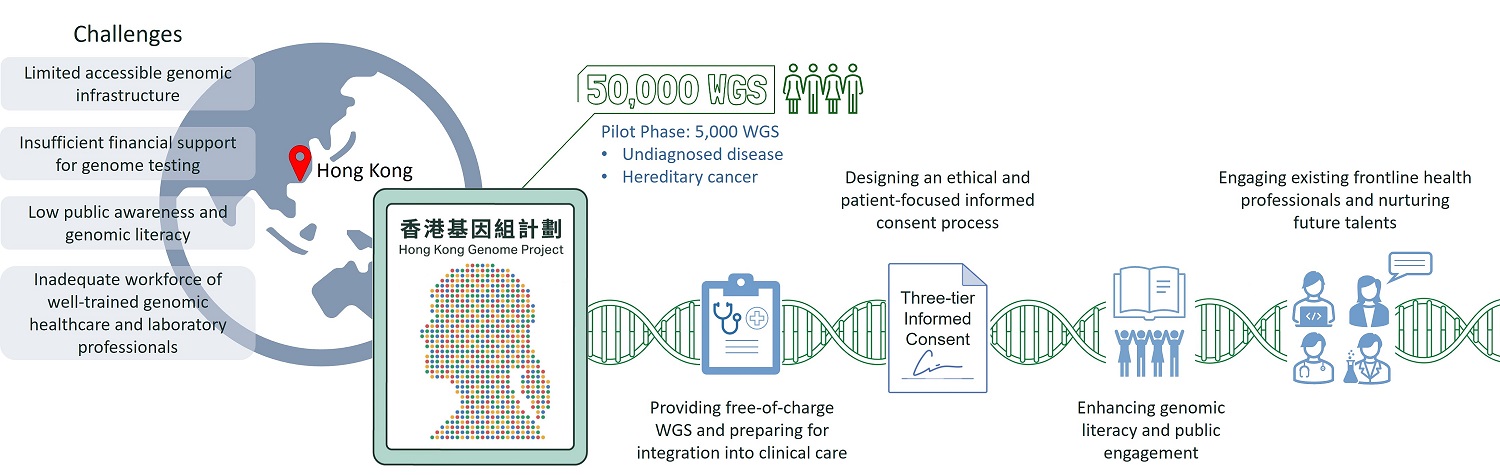
Potentials and challenges of launching the pilot phase of Hong Kong Genome Project
Abstract
Genomic medicine and precision medicine initiatives have taken centre stage in scientific, clinical, as well as health economics and utility research on the global scene for the past decade. It is clear the important role genomic advancement has played in enhancing diagnostic rate, streamlining personalised treatment, and improving efficacy of the overall clinical management of undiagnosed, rare, and common diseases for humankind. The Hong Kong Genome Institute (HKGI) was established in May 2020 within the Food and Health Bureau, Hong Kong Special Administrative Region, to integrate genomic medicine into mainstream healthcare. The main goals of setting up HKGI are to (1) improve the diagnostic rate and future care for individuals affected by undiagnosed diseases and hereditary cancers using whole genome sequencing; (2) advance research in genomic science; (3) nurture talents in genomic medicine; and (4) enhance public genomic literacy and overall engagement through the launching of the Hong Kong Genome Project (HKGP). In this paper, we review the current landscape and specific challenges encountered during the construction of the infrastructure and implementation of the pilot phase of HKGP. Through reviewing what has been achieved and established to date, and the potentials and prospects that have emerged in the process, this paper will provide insights into planning the main phase of HKGP, and considerations for our international counterparts when building similar projects.
Keywords
INTRODUCTION
The rapid advancement in genomic medicine has offered huge potential and opportunities in accurate diagnosis, personalised treatment, and efficacious surveillance and prevention of both undiagnosed, rare, and common diseases. Since the completion of the renowned Human Genome Project in 2003, progresses in next-generation sequencing and bioinformatic technologies have accelerated the execution of large-scale human genome projects.
A growing number of countries have invested immense resources to utilise the human genome in view of the importance of integrating genomic medicine into future medical and health development[1]. Precision medicine initiatives involving sequencing of thousands to millions of human genomes are being launched worldwide, and experiences are shared via publications and exchanges in international conferences. The largest projects in terms of scale are the 1+ Million Genomes Initiative, which is a collaboration among 24 European Union countries; and the All of Us Research Program of the United States (US), both aim at recruiting and analysing genomes of one million participants[1].
Many of these genomic initiatives strategically focus on rare disease patient cohorts [Table 1]. Affected individuals are often difficult to diagnose and have very long diagnostic journeys, and thus they are more likely to benefit from comprehensive and unbiased genome sequencing. Studies did not only show the diagnostic and clinical utility of genome sequencing in rare disease cohorts in ending the diagnostic odyssey at a personal level, but there is also the extended impact on the social and health economics aspects[2-4].
Examples of large-scale genomic projects with target size larger than 20,000 genomes include patients with rare and undiagnosed diseases
| Country | Project/institute name | Target size | Years active | Status | Genomic sequencing modality |
| Australia | Australian Genomics Health Alliancei | > 25,000 | 2016-ongoing | Active | Depending on the flagship projects |
| Australia | Genomics Health Futures Missionii | 200,000 | 2018-ongoing (targeted completion in 2028) | Active | Depending on the projects |
| China | China Precision Medicine Initiativeiii | 100,000,000 | 2015-ongoing (targeted completion in 2030) | Active | WGS |
| Denmark | Danish National Genome Centeriv | 60,000 | 2021-ongoing (targeted completion in 2024) | Active | WGS |
| France | France Genomic Medicine 2025v | 235,000 per annum | 2015-ongoing (targeted completion in 2025) | Active | WGS/WES/RNA seq |
| Hong Kong | Hong Kong Genome Projectvi | 50,000 | 2021-ongoing (targeted completion in 2026) | Active | WGS |
| Japan | GEnome Medical alliance Japanvii | Nationwide | 2018-ongoing | Active | WGS |
| Saudi Arabia | Saudi Human Genome Programviii | 100,000 | 2018-ongoing (targeted completion in 2030) | Active | WGS/WES/panel |
| Thailand | Genomics Thailand Initiativeix | 50,000 | 2019-ongoing (targeted completion in 2024) | Active | WGS/WES/Microarray |
| Turkey | Turkish Genome Projectx | 100,000 | 2017-ongoing (targeted completion in 2023) | Active | WGS |
| United Kingdom | 100,000 Genomes Projectxi | 100,000 | 2013-2018 | Completed | WGS |
| United Kingdom | Our Future Healthxii | 5,000,000 | 2020-ongoing | Active | Depending on the projects |
| United States | NHGRI Centers for Mendelian Genomicsxiii | Nationwide | 2011-ongoing | Active | Depending on the projects |
| United States | NIH Undiagnosed Diseases Programxiv | Nationwide | 2008-ongoing | Active | WES/Microarray |
These initiatives have facilitated the reform of healthcare in numerous areas. For example, the 100,000 Genomes Project led by Genomics England has proven to be a well-recognised success, based not only on their gene discoveries and scientific publications, but also by their impact on the nation’s healthcare ecosystem[5]. The Project has enabled and driven the necessary changes and reform in the National Health Service (NHS) by offering whole-genome sequencing as routine.
Challenges and barriers of genetic services development: global and local perspectives
Although countries such as the United Kingdom, US, and China strive to be global leaders by launching ambitious population-based genome sequencing projects, experts in the field who are heavily involved pointed out that many overlooked the importance of understanding and managing the “public appetite” while designing the projects[6]. In an era in which the public highly values data security and personal privacy, public trust remains a universal challenge for implementing any genome initiatives and campaigns.
Albeit the difficulties and uncertainties in managing public trust and confidence, the design of these national whole-genome sequencing projects shares one humane mission - the elimination of the major barriers to accessing genomic services. Cost and limitations of an accessible service infrastructure for targeted patients have remained significant hurdles to genomics and genetics services worldwide.
Prior studies pinpointed patients’ difficulties in getting proper and timely referrals to genetic testing services (for rare, undiagnosed, or hereditary cancer syndromes) as a universal obstacle. Pragmatic difficulties in many institutions lie in the lack of well-established multi-disciplinary genetic clinics and clearly delineated triage system for managing referrals and arranging timely genetic consultation[7-9].
Besides the lack of access to well-trained clinical laboratory and healthcare professionals, the Asian socio-cultural beliefs of families and genetics are also major impediments in the Asian region such as Singapore[10], Malaysia[11] and Hong Kong[12]. Perceived difficulties and inadequateness do not only originate from patients. Studies also showed that frontline clinicians felt unequipped to make accurate referrals[13-15]. A recent local study on the attitudes and clinical practices of primary care physicians (PCPs) showed that 68% (104/151) of the surveyed PCPs in both public and private service organisations did not know the referral pathway for patients with suspected or confirmed genetic disorders[16].
While genetic testing is a critical part of diagnostic journeys of patients with rare disease and cancer, cost remains a critical hurdle in genetic services in Hong Kong. As the public service only sponsors and covers essential healthcare for all, a limited number of genetic tests are covered[12,17]. Private financing schemes, including employer-based and privately purchased insurance as well as household out-of-pocket payment, represent common forms of healthcare financing in Hong Kong. As health insurance is not mandatory, 49.2% of the people in Hong Kong are not covered by any form of medical insurance and many would have to bear the cost of genetic and genomic tests out-of-pocket[18]. In a local study investigating the cost-effectiveness of using chromosomal microarray as the primary test for prenatal diagnosis, only 41.8% (300/717) of pregnant women were willing to pay fully out-of-pocket to undergo the test. In scenarios where the test was subsidised at an increasing portion, more women were willing to undergo and benefit from the diagnostic test[19]. Another local study on Chinese females at-risk of Hereditary Breast and Ovarian Cancer Syndromes showed that sponsored genetic counselling and testing were crucial for them as the majority would not have opted for self-financed testing[20].
Some Asian countries have also started national genome projects to advance genomic medicine [Table 1]. A fundamental change and reform of the current genomic landscape is undoubtedly an essential first step in expediting the advancement in precision medicine. Without that, it is difficult to set the stage to facilitate the breakthrough in improving the diagnostic rate and clinical management of rare and undiagnosed diseases.
The characteristics of Asian robust city - Hong Kong
Chinese represents the largest population in the world, and Hong Kong has become a Special Administrative Region of the People’s Republic of China since 1997. As a world recognised financial centre, with a modern city standard and quality of living, Hong Kong retains its own economic, legal, social, healthcare, and welfare infrastructures.
According to the latest population projections announced by the Census and Statistics Department (C&SD)[21], Hong Kong’s population is projected to increase from 7.51 million in 2019 to 8.10 million in 2039. With reference to the 2016 by-census[22], over 90% of the Hong Kong population is ethnic Chinese and other ethnic groups constitute the remaining 8% of the population. With a relatively homogenous population, Hong Kong is therefore often being seen as a strategic location to conduct health-related studies on Southern Chinese populations.
Current landscape of genetics and genomics in Hong Kong
Hong Kong has a well-established dual-track healthcare system comprised of 43 (and growing) public hospitals managed by the Hospital Authority. Majority of the population (around 85%) uses public healthcare, while the remaining population has convenient access to self-financed medical services. The present paper systematically explores the history and background of the genomic and genetic services in the context of the existing health service landscape, which serves as the backbone of the strategy and implementation of the first large-scale genome project - The Hong Kong Genome Project (HKGP).
Hong Kong is not building genomics and genetics work from ground zero. Clinicians at the Department of Health (the Government's health adviser and agency which executes health policies and statutory functions), along with the Hospital Authority, medical schools of universities, and private hospitals, have been providing continuous clinical genetics service and research support to the public in Hong Kong for decades.
For historical reasons, Clinical Genetic Services of the Department of Health provides the majority of public diagnostic and counselling services to families with inherited genetic disorders. These services include diagnosis of genetic disorders, genetic screening, genetic counselling, and genetic testing in relation to disease management. Post-test clinical management and periodic screening have usually been taken up by Hospital Authority via its public hospitals’ infrastructure. Local academic researchers and scientists, usually with a job-induced passion, opportunities, and research/charitable fundings, have attained internationally recognised achievements in genomic research. As mentioned above, the majority of the patients access genetic services provided by the Department of Health or Hospital Authority in Hong Kong. Since only essential investigations are covered, advanced genomic tests such as genome sequencing are either sent to overseas clinical laboratories with patient bearing the full cost or done in a research setting[12].
Despite the hard work and contributions by various parties, the medical-academic-scientific fields in Hong Kong have identified enduring issues with the above set-up and workflow. The genomic services provided by the two medical schools are often research-based and lack the capacities to be used as routine clinical services. The Government acknowledged in the policy address of 2017 that there was an urgent need to explore a more standardised and better co-ordinated clinical pathway, invest in the training and nurturing of related professionals, and improve the overall management of genetic and genomic services in Hong Kong. A Steering Committee was thus formed to review the landscape of genetics and genomics in Hong Kong and map out the strategies for developing genomic medicine.
Steering committee recommendations
After reviewing the experiences of other national sequencing projects and deliberating the local environment, the Steering Committee published its report and proposed the eight recommendations to promote local development and integration of genomics into our healthcare system[12].
1. Launching the HKGP
2. Enhancing clinical services in genetics and genomics
3. Nurturing talents in genomic medicine
4. Enhancing public engagement in genomic medicine
5. Enhancing the laboratory network with effective referral mechanism and centralisation of advanced genetic and genomic tests
6. Facilitating the establishment of a biobank network for genomic research
7. Enhancing the regulation on use of genetic data for insurance and employment purposes
8. Promoting the proper use of genetic and genomic tests
THE LAUNCH OF HONG KONG GENOME PROJECT: PILOT PHASE
Based on the recommendations from the Steering Committee, the Government set up the Hong Kong Genome Institute (HKGI), a company wholly owned by the Government, to implement the HKGP in partnership with the Food and Health Bureau, Department of Health, Hospital Authority, and local universities. The Government reserved HKD$1.2 billion (USD$150 M) for the Project, the first population-scale whole-genome sequencing project in Hong Kong with the aim to sequence up to 50,000 genomes by 2025.
Leveraging the experience from other sequencing projects, the HKGP is expected to serve as a catalyst to advance the development of genomic medicine in Hong Kong. In addition to the clinical benefits, it would also strengthen the local talent pool, establish infrastructure and protocols, and drive new policy measures in the field of genomic medicine. The data generated from HKGP would form one of the largest health-related local databases and create new research opportunities for studying various diseases.
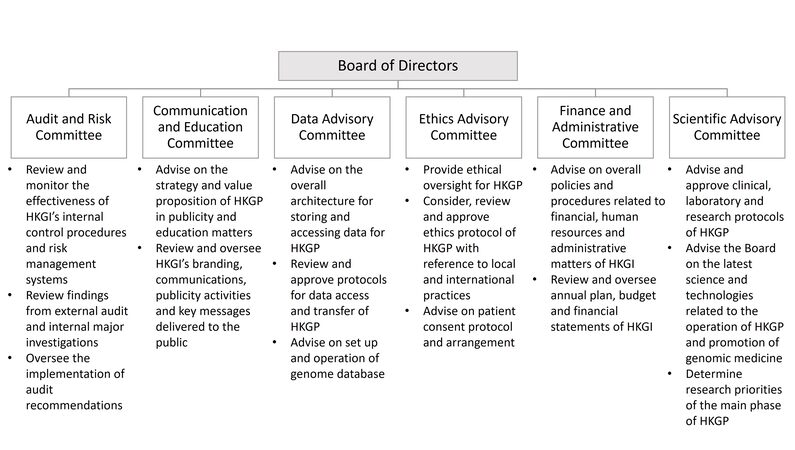
Since its incorporation in May 2020, the HKGI has been fully engaged in recruiting professionals in the field and setting up various hardware and software necessary for implementing the HKGP. It would pioneer the development of a clinical genomic platform for the population of Hong Kong, with continuing contributions from all members of HKGI under the governance of six committees, overseen by the Board of Directors [Figure 1].
Figure 1. Governance of the Hong Kong Genome Institute. HKGI is overseen by the Board of Directors and six Advisory Committees for effective implementation of HKGP. HKGI: Hong Kong Genome Institute; HKGP: Hong Kong Genome Project.
The implementation of HKGP is being conducted in two phases, namely the pilot phase and the main phase. Under the pilot phase of the Project, approximately 2000 cases (~5000 genomes, as majority of the cases will be subjected to trio analysis) will be sequenced. The recruitment eligibility criteria include patients with undiagnosed diseases, hereditary cancers (genetic predisposition to cancer), and their family members. During the short-term and long-term strategic planning process, the current landscape of genomics and genetic services in Hong Kong was carefully reviewed to identify key issues and challenges facing HKGI in the future. Lessons learnt during the pilot phase would guide the directions of the Project’s main phase as it grows and expands its coverage into other disease areas and research cohorts. The main phase will sequence approximately 18,000 cases (~45,000 genomes).
Participants enrolled in the HKGP receive WGS analysis in addition to the usual clinical care. A trio-based approach would be adopted in majority of the cases, where feasible, to assist genomic data interpretation. Phenotypic data is recorded using Human Phenotype Ontology (HPO) terms to standardise clinical data input. A whole blood sample is collected from all participants for DNA extraction and subsequent WGS analysis in the HKGI laboratory and sequencing centre at a public-funded university. For participants with suspected hereditary cancers, an additional tumour sample, preferably fresh frozen sample, could be obtained to compare against the germline genome for the detection of somatic variants.
Engagement with frontline healthcare and medical-academic systems
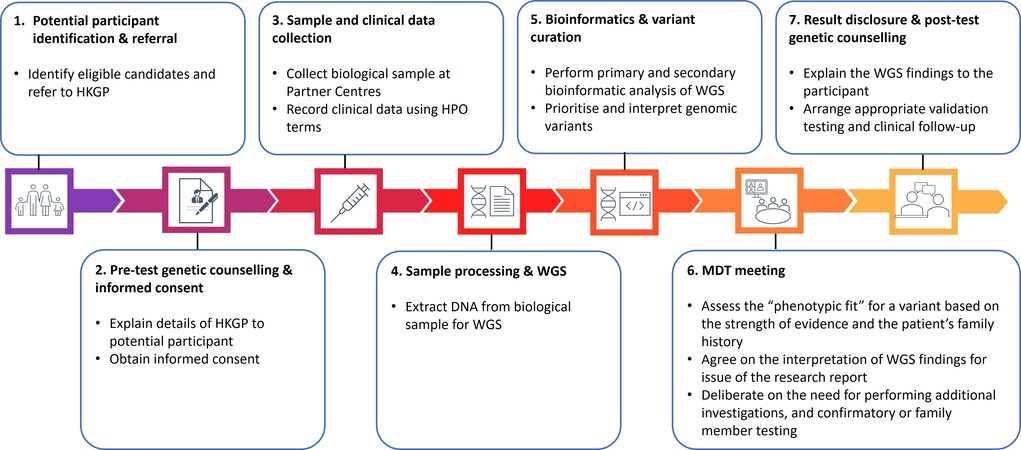
The recruitment structure of HKGP is built on the establishment of partnerships with existing healthcare systems. For the pilot phase, HKGI has established and fully funded the operations of three HKGP Partnering Centres at the university-affiliated teaching hospitals within the public healthcare system to ensure equitable access to the Project. The Partnering Centres play important roles in providing clinical support, such as recruiting participants, providing genetic counselling, collecting samples, handling enquiries, delivering genome sequencing results, and liaising with clinical teams of the hospitals [Figure 2]. The Partnering Centres’ participation in HKGP not only introduces whole-genome sequencing (WGS) in the public healthcare system, but also offers a unique opportunity to provide experiential learning and training for the workforce in the Partnering Centres, pathing the way for clinical implementation of genomic medicine.
Genome project for the people: public trust and confidence
As mentioned above, public trust and understanding of genomics unquestionably remain a global challenge to the success of all genomics campaigns. With the aim to facilitate public awareness and understanding, the HKGP spearheads the development of guidelines and standardised protocols on informed consent and the collection, storage and sharing of genomic data in a secure, ethical, and responsible manner.
HKGI respects and values the public opinions and perception of the HKGP and carried out three focus groups (undiagnosed disease patient groups, hereditary cancer patients and family members, and clinicians) to understand the people’s general opinions on genomic testing and studies. A recurring theme mentioned in the focus groups was the emphasis on making the process as “transparent” and “fully informed” as possible. The findings were highly valued and shaped the current structure of the informed consent and patient recruitment process, publicity, and public engagement strategies of the Project.
For genomic medicine to be widely adopted, there is also a need for public engagement in the areas of science and medicine to improve genomic literacy and foster public support. The Project provides a good opportunity to enhance the public’s understanding of the benefits and limitations of genomic medicine and genome sequencing on healthcare decisions, as well as relevant ethical and privacy standards. To complement and promote the launch of HKGP, a dedicated project website with user-friendly information, videos, and publications on genomic medicine are developed to draw public’s interest in genomics and enhance genomic literacy (Available from: https://hkgp.org/en/). With the governance of its Communications and Education Committee [Figure 1], HKGI will continue to do pulse checks on all public engagement and awareness strategies adopted by the Project.
Other than project marketing and promotion strategies to manage public opinions, high-level of transparency, clear, and continuous communication between healthcare professionals and patients is a crucial pillar of building public trust and credibility[23]. One major priority in the implementation of HKGP is the design of a patient-focused and ethical consent protocol. A truly thorough “informed consent” practice for complicated WGS testing must be done in a thorough, colloquial, non-directional, and humane manner.
The thorough process in developing a patient-focused consent protocol
In the era of genomic sequencing, the models of consent have stretched the traditional approaches as a vast amount of genetic information can be obtained from a single test. The expanded scope of testing puts pressure on the width and depth of pre-test discussion on several emerging areas, including but not limited to genomic data handling and privacy, uncertain results, unexpected or secondary findings and the associated psychosocial and familial impact. There is still a lack of consensus in the genetic community about the standard constitution of informed consent in genomic settings owing to its complexity.
During the design process of HKGP consent protocol, consent protocols of other large-scale genomic projects such as the 100,000 Genomes Project, the latest consent policy and consent clauses for genomic research from the Global Alliance for Genomics and Health (GA4GH) were referenced, with the understanding that these frameworks have considered the opinions of the professionals and public[24-27]. To address the specific project details of HKGP, the Ethics Advisory Committee (EAC) [Figure 1] of HKGI advises on ethical issues in relation to the design and implementation, including the endorsement of the consent protocol of HKGP. The formation of EAC comprises external experts and professionals from diverse backgrounds to offer crucial perspectives on relevant ethical decisions, including bioethicists, lawyers, patient group representatives, and clinical geneticists. Opinions from the participating local bodies, including the Equal Opportunities Commission, Privacy Commission for Personal Data, and legal advisers, are gathered in addition to comments from the EAC to ensure concerns from other stakeholders are well-addressed before the submission to the Institutional Review Boards (IRB).
One of the challenges of achieving informed consent for genomic sequencing is often related to the subjective and poorly defined line of adequate information and comprehension[28]. With an aim to meet the specific needs of individuals and facilitate the consent process, the consent materials of HKGP adopted a colloquial and well-organised presentation according to carefully designed sequences and levels of detail. The consent form seeks broad consent for using participants’ donated samples, collecting clinical information for genetic analysis, and sharing de-identified data for approved research studies. It also provides options for participants to receive secondary findings or to provide contact information for participation in future research outside the scope of HKGP. For participants who consented to receive secondary findings, HKGP will return pathogenic/likely pathogenic variants in a well-defined list of genes that are in line with the international practice. Thirteen genes are currently included in the analysis and the reporting approach is age-dependent [Table 2]. While the gene list and approach will be reviewed periodically, participants are informed of the scope and practice during the consent process and have access to the gene list through our website.
List of secondary findings reported by the Hong Kong Genome Project
| Conditions | Genes | Adults | Children |
| Lynch syndrome | MLH1 | √ | x |
| MSH2 | √ | x | |
| MSH6 | √ | x | |
| Familial adenomatous polyposis | APC | √ | √ |
| MYH-associated polyposis | MUTYH | √ | x |
| Hereditary breast and ovarian cancer syndrome | BRCA1 | √ | x |
| BRCA2 | √ | x | |
| Von Hippel-Lindau syndrome | VHL | √ | √ |
| Multiple endocrine neoplasia | MEN1 | √ | √ |
| RET | √ | √ | |
| Familial hypercholesterolaemia | LDLR | √ | √ |
| APOB | √ | √ | |
| PCSK9 | √ | √ |
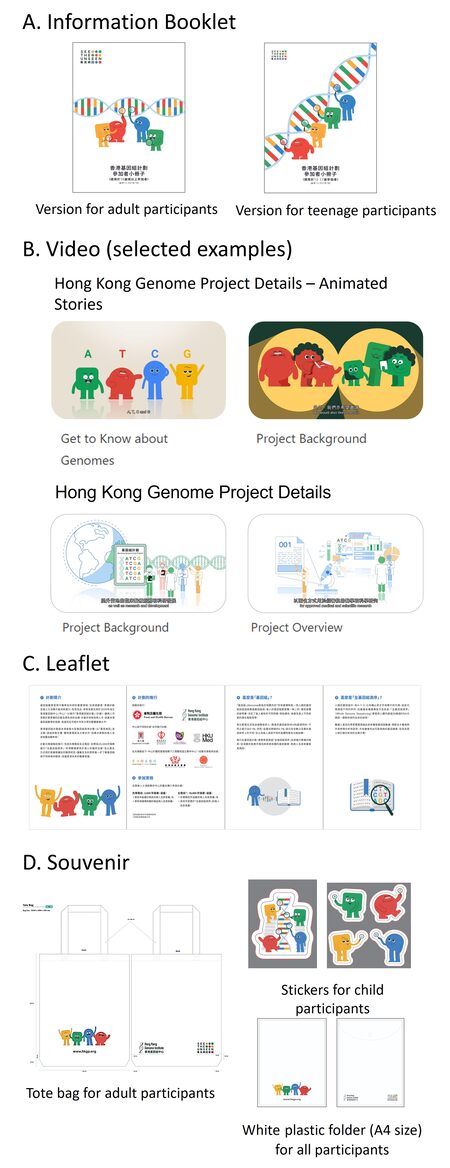
Apart from the consent form, two versions of information booklets and 11 videos are produced to provide detailed and step-by-step information for potential participants [Figure 3]. Since the potential participants are from a wide age range, including adults, teenagers, and children, the entire content is presented in age-appropriate and colloquial dialects and enriched with coloured infographics and animated cartoons to facilitate easy understanding of informed consent.
Figure 3. Informational and promotional materials for Hong Kong Genome Project. Cartoon characters that represent the four nucleotide bases in human genome are included in all materials as mascots of the Project. (A) Information Booklet (available in Traditional Chinese and English). Versions with different levels of detail and styles of language are prepared for adult and teenage participants aged 12-17, respectively. (B) Examples of video on HKGP webpage (bilingual subtitles in Traditional Chinese and English). (C) Leaflet (available in Traditional Chinese and English). (D) Souvenir.
As emphasised, public trust and confidence in genomic testing are vital of the success of any large-scale genomic project. Background of the Project, recruitment information and consent materials can be easily accessed via HKGP website. This also helps set the inaugural move in enhancing public literacy and promoting awareness of genomic medicine in Hong Kong.
Three-tier informed consent process
HKGP recruits participants of all ages and different versions of consent forms are formulated to customise their needs. Like any research involving child participants, their parent or legal guardian is involved in the consent process and will provide written consent on the child’s behalf as a proxy. While obtaining assent is a universal aim in both clinical and research consent, there is no single practice internationally. The concept of assent and its application varied in different countries, ranging from the most paternalistic approach of adhering strictly to legal provisions to the most liberal approach of respecting child/young participants’ wishes as much as possible.
A unique three-tier model of informed consent was designed and implemented in the HKGP after thorough discussions at EAC [Table 3]. The model is adopted to delineate the consent and assent rationale to balance the paternalistic and liberal approaches in the international arena. In Hong Kong, the legal age is 18 years old; therefore, adult participants aged 18 or above would sign the consent form themselves. Participants aged 16 and 17 would co-sign their consent form with parents/legal guardians. This approach aims at recognising and maximising the autonomy and best interests of adolescents who have sufficient capacity to understand the Project entirely. If there is any difference in opinions between the participants and their parents/legal guardians, further discussion would be encouraged, and the participants would not be recruited. For participants aged below 16, their consent forms would be signed by parents/legal guardians. Verbal assent would be taken wherever possible, in accordance with the child participant’s level of understanding. When child participants reach legal age, a formal re-consent process will be conducted.
The three-tier model of informed consent of Hong Kong Genome Project
| Category | Age (years) | Consent | Assent |
| Adult participants | ≥ 18 | Signed by participant | Not applicable |
| Child participants | 16 to 17 | Signed by parent/legal guardian and co-sign by child participant | Materials aiding assent process |
| < 16 | Signed by parent/legal guardian | Materials aiding assent process or verbal assent (whenever possible) |
The entire consent, pre-test counselling, and withdrawal process (if applicable) of HKGP are based on this three-tier model, with an emphasis on open, pro-active, and respectful engagement of all participants about their involvement in the Project in an age-appropriate, humane, and ethical manner.
Needs, challenges, and aspirations for developing the genetic counselling profession in Hong Kong
The previous section depicted the importance of informed consent and pre-test genetic counselling as the first step of patient engagement of HKGP. The process is performed by genetic counsellors specifically hired and trained to execute this Project.
Genetic counselling is a relatively novel and unorganised profession in Hong Kong. Our existing genetic counselling related services have been supported by a group of dedicated and on-the-job trained frontline medical personnel (with or without an overseas board-certified qualification). As the demand for genomic and genetic services has expanded rapidly in the past decade, there is an urgent need to standardise the genetic counselling practice and facilitate training in Hong Kong.
One of the prevailing challenges is the lack of accredited programmes and Board to train and register new genetic counsellors or those who are practising in the field. This further exacerbates the insufficient pool of professional genetic counsellors to meet the rapid growth in service demands in Hong Kong. There is also a pressing need to delineate the scope of practice, code of ethics, and quality assurance of the industry. Without the start of a blueprint or a governing body, it is difficult to plan and implement continuous training and development of genetic counselling practice.
HKGP acts as a catalyst and sets the stage in providing a platform to nurture a group of genetic counsellors by providing funding and resources for Partnering Centres to hire designated genetic counsellors. Through enabling the role of genetic counsellors in the operational workflow, awareness and knowledge amongst other healthcare professionals concerning the scope of practice of genetic counsellors can be enhanced.
Learning opportunities for genomic professionals
Other than paving the way for the genetic counsellors, HKGP also serves as an important platform to provide continuous enrichment to clinicians, laboratory scientists, bioinformaticians, genomic variant curators, nurses and trainees. The Project offers experiential learning opportunities to different specialities in a collaborative environment.
One of the challenges of working with various professionals from a multi-disciplinary background is the lack of a common language; it is understandable that specialities may use different vocabularies to provide descriptions of the same observed phenotype. Hence, HPO has become the standard for phenotype exchange in the age of genomic medicine, describing human phenotypes systematically and enabling computational inference in genotype-phenotype analyses[29]. It has been used extensively to support diagnostic interpretation of genomic variations in rare diseases and adopted by many large-scale genome projects such as the 100,000 Genomes Project and the Undiagnosed Disease Network[30]. In alignment with international standards, the HKGP also adopts the use of HPO terms to record clinical features, facilitating effective communication amongst professionals of diverse backgrounds.
Apart from using HPO as the “common language” amongst professionals and the computational analysis system, HKGP takes on a multi-disciplinary approach in achieving the project outcomes. Post-analysis multi-disciplinary team (MDT) meeting is one of the core components of the HKGP workflow, allowing inputs from relevant specialists to achieve consensus on the genetic diagnosis and patient’s management plan. For example, genomic variant curators will work closely with the referring clinicians to determine the cases that would require further in-depth discussion in an MDT meeting, where the referring clinician will present phenotypes of the participant and the genome variant curators will present the variant finding and interpretation.
Further to providing means of communication for a diverse group of professionals, we hope HKGP serves as an active, experiential learning platform to enable members and trainees to appreciate and familiarise the integration of genomic medicine into practices.
CONCLUSION
HKGI takes on the pioneering role in developing genomic medicine in Hong Kong. Through the launch and implementation of HKGP, it aims to overcome the major hurdles of cost and lack of access to WGS in the existing services. It provides free-of-charge WGS for individuals and families suffering from the clinical odyssey of rare diseases, hereditary cancers, and other undiagnosed diseases. Since HKGP is the first large-scale genome project in Hong Kong, it lays the groundwork for developing the infrastructure and workflow to prepare for the integration of genomic medicine into routine clinical care in the foreseeable future. It also equips the existing workforce, trains the next-generation of genomic experts, and prepares the general public for the age of precision medicine.
With the tight timeline to recruit and analyse an ambitious number of genomes, this is only the beginning of a challenging and farsighted mission. To measure the success of the programme, a panel of international scientists will evaluate the structure, process and outcome of the HKGP independently. We look forward to sharing the evaluation outcome and other progress of the Project as separate publications and case reports. With the perseverance, unyielding efforts, and dedication of all the stakeholders, we aim to live up to our vision “To avail genomic medicine to all for better health and well-being”.
DECLARATIONS
AcknowledgmentsWe would like to thank all members of the HKGI in preparing the launch of the HKGP. This work would not have been possible without the instrumental leadership and guidance from Dr Su-vui Lo, Chief Executive Officer. We extend our gratitude to the Board of Directors and Advisory Committees for their continuous support and advice. We also wish to acknowledge the support of the HKGP stakeholders: the Food and Health Bureau; Hospital Authority; Department of Health; and Partnering Centres at The University of Hong Kong/Queen Mary Hospital, The Chinese University of Hong Kong/Prince of Wales Hospital, and Hong Kong Children's Hospital.
Authors’ contributionsContributed intellectually and practically to this piece: Chu ATW, Fung JLF, Tong AHY, Chow SM, Chan KYK, Yeung KS, Lo HM, Hong Kong Genome Project, Chung BHY
Availability of data and materialsNot applicable.
Financial support and sponsorshipNot applicable.
Conflicts of interestAll authors declared that there are no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Chung BHY, Chau JFT, Wong GK. Rare versus common diseases: a false dichotomy in precision medicine. NPJ Genom Med 2021;6:19.
3. Stark Z, Schofield D, Martyn M, et al. Does genomic sequencing early in the diagnostic trajectory make a difference? Genet Med 2019;21:173-80.
4. Wise AL, Manolio TA, Mensah GA, et al. Genomic medicine for undiagnosed diseases. Lancet 2019;394:533-40.
6. Wheway G, Mitchison HM, Ambrose JC, et al. Opportunities and challenges for molecular understanding of ciliopathies - the 100,000 genomes project. Front Genet 2019;10:127.
7. Swanson CL, Kumar A, Maharaj JM, et al. Increasing genetic counseling referral rates through bundled interventions after ovarian cancer diagnosis. Gynecol Oncol 2018;149:121-6.
8. Dragojlovic N, Borle K, Kopac N, et al. The composition and capacity of the clinical genetics workforce in high-income countries: a scoping review. Gene Med 2020;22:1437-49.
9. Armel SR, Volenik A, Demsky R, Malcolmson J, Maganti M, McCuaig J. Setting a baseline: A 7-year review of referral rates and outcomes for serous ovarian cancer prior to implementation of oncologist mediated genetic testing. Gynecol Oncol 2020;158:440-5.
10. Chiang J, Ngeow J. The management of BRCA1 and BRCA2 carriers in Singapore. Chin Clin Oncol 2020;9:62.
11. Shaw T, Ishak D, Lie D, et al. The influence of Malay cultural beliefs on breast cancer screening and genetic testing: a focus group study. Psychooncology 2018;27:2855-61.
12. Steering Committee of Genomic Medicine, Hong Kong Special Administrative Region. Strategic development of genomic medicine in Hong Kong; 2020. Available from: https://www.fhb.gov.hk/download/press_and_publications/otherinfo/200300_genomic/SCGM_report_en.pdf [Last accessed on 9 Jun 2022].
13. Zhao X, Wang P, Tao X, Zhong N. Genetic services and testing in China. J Community Genet 2013;4:379-90.
14. Hegarty J, Egan SM, Jones MM, et al. The unmet need in cancer genetic services: conducting an environmental scan of the cancer genetics services in an Irish context underpinned by a mixed methods approach- report prepared for the Irish cancer society, Ireland. 2021. Available from: https://www.cancer.ie/sites/default/files/2021-04/Conducting%20an%20environmental%20scan%20of%20the%20cancer%20genetics%20services%20Report%202021.pdf [Last accessed on 9 Jun 2022].
15. Truong TK, Kenneson A, Rosen AR, Singh RH. Genetic referral patterns and responses to clinical scenarios: a survey of primary care providers and clinical geneticists. J Prim Care Community Health 2021;12:21501327211046734.
16. Yu MWC, Fung JLF, Ng APP, et al. Preparing genomic revolution: attitudes, clinical practice, and training needs in delivering genetic counseling in primary care in Hong Kong and Shenzhen, China. Mol Genet Genomic Med 2021;9:e1702.
17. Hospital Authority, Hong Kong Special Administrative Region. Strategic service framework for genetic and genomic services; 2019. Available from: https://www.ha.org.hk/haho/ho/ap/HAGGSSSF_Eng.pdf [Last accessed on 9 Jun 2022].
18. Census and Statistics Department, Hong Kong Special Administrative Region. Thematic Household Survey Report No. 74.; 2021. Available from: https://www.censtatd.gov.hk/en/data/stat_report/product/C0000022/att/B11302742021XXXXB0100.pdf [Last accessed on 9 Jun 2022].
19. Chung CCY, Chan KYK, Hui PW, et al. Cost-effectiveness analysis of chromosomal microarray as a primary test for prenatal diagnosis in Hong Kong. BMC Pregnancy Childbirth 2020;20:109.
20. Chu A, Man-Sik Tse D, To Ki Suen D, Kwong A. Baseline knowledge and receptiveness to genetic testing for hereditary breast and ovarian cancer syndromes in Chinese high-risk females. J Commun Gene 2021.
21. Census and Statistics Department, Hong Kong Special Administrative Region. Hong Kong Population Projections: 2020-2069; 2020. Available from: https://www.censtatd.gov.hk/en/data/stat_report/product/B1120015/att/B1120015082020XXXXB0100.pdf [Last accessed on 9 Jun 2022].
22. Census and Statistics Department, Hong Kong Special Administrative Region. 2016 population by-census: summary results; 2017. Available from: https://www.bycensus2016.gov.hk/data/16bc-summary-results.pdf [Last accessed on 9 Jun 2022].
23. Dheensa S, Samuel G, Lucassen AM, Farsides B. Towards a national genomics medicine service: the challenges facing clinical-research hybrid practices and the case of the 100 000 genomes project. J Med Ethics 2018;44:397-403.
24. Consent Clauses for Genomic Research. Consent clauses for genomic research. 2020. Available from: https://drive.google.com/file/d/1O5Ti7g7QJqS3h0ABm-LyTe02Gtq8wlKM/view [Last accessed on 9 Jun 2022].
25. Global Alliance for Genomics and Health. Global Alliance for genomics and health: consent policy (v2.0); 2019. Available from: https://www.ga4gh.org/wp-content/uploads/GA4GH-Final-Revised-Consent-Policy_16Sept2019.pdf [Last accessed on 9 Jun 2022].
27. Genomics England. Consent in the 100,000 genomes project. Available from: https://www.genomicsengland.co.uk/about-genomics-england/the-100000-genomes-project/information-for-gmc-staff/consent/ [Last accessed on 9 Jun 2022].
28. Rego S, Grove ME, Cho MK, Ormond KE. Informed consent in the genomics era. Cold Spring Harb Perspect Med 2020;10:a036582.
29. Robinson PN, Köhler S, Bauer S, Seelow D, Horn D, Mundlos S. The human phenotype ontology: a tool for annotating and analyzing human hereditary disease. Am J Hum Genet 2008;83:610-5.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Chu ATW, Fung JLF, Tong AHY, Chow SM, Chan KYK, Yeung KS, Lo HM, Hong Kong Genome Project, Chung BHY. Potentials and challenges of launching the pilot phase of Hong Kong Genome Project . J Transl Genet Genom 2022;6:290-303. http://dx.doi.org/10.20517/jtgg.2022.02
AMA Style
Chu ATW, Fung JLF, Tong AHY, Chow SM, Chan KYK, Yeung KS, Lo HM, Hong Kong Genome Project, Chung BHY. Potentials and challenges of launching the pilot phase of Hong Kong Genome Project . Journal of Translational Genetics and Genomics. 2022; 6(2): 290-303. http://dx.doi.org/10.20517/jtgg.2022.02
Chicago/Turabian Style
Chu, Annie T. W., Jasmine L. F. Fung, Amy H. Y. Tong, Sin Man Chow, Kelvin Y. K. Chan, Kit San Yeung, Hei Man Lo, Hong Kong Genome Project, Brian H. Y. Chung. 2022. "Potentials and challenges of launching the pilot phase of Hong Kong Genome Project " Journal of Translational Genetics and Genomics. 6, no.2: 290-303. http://dx.doi.org/10.20517/jtgg.2022.02
ACS Style
Chu, ATW.; Fung JLF.; Tong AHY.; Chow SM.; Chan KYK.; Yeung KS.; Lo HM.; Hong Kong Genome Project.; Chung BHY. Potentials and challenges of launching the pilot phase of Hong Kong Genome Project . J. Transl. Genet. Genom. 2022, 6, 290-303. http://dx.doi.org/10.20517/jtgg.2022.02
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 34 clicks
Cite This Article 34 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.