NUT midline carcinoma presenting initially as thyroid cancer: a case report and review of treatment
Abstract
The purpose of this abstract is to provide data on a 27-year-old woman with a rare and rapidly progressive cancer, Nuclear in Testis (NUT) carcinoma (NC), and to highlight possible treatment options for her specific gene translocation of NSD3-NUT. To our knowledge, this is the first case in the literature that was presented initially as anaplastic thyroid cancer. We propose that targeted therapy with a histone lysine methyltransferase inhibitor may be of benefit as adjunctive therapy in patients with the NSD3-NUT gene translocation.
Keywords
INTRODUCTION
Cancer of unknown primary site (CUP) is a heterogeneous group of metastatic cancers for which a primary tumor cannot be identified after a standardized work-up at the time of diagnosis. Though its incidence is in decline, it remains surprisingly common, with historical series estimating an incidence of 3%-5% of invasive cancer diagnoses; with the advent of newer diagnostic technologies, the incidence rate of CUP has decreased to 1%-2%[1]. It is estimated by the American Cancer Society that in 2022, approximately 30,620 cases of CUP will be diagnosed in the United States[2]. An accurate diagnosis is essential because tumor biomarker-targeted treatment can impact survival.
Nuclear protein in testis (NUT) carcinoma (NC), also known as NUT-midline carcinoma, is a rare and nearly universally fatal malignancy that primarily affects children and young adults; it typically presents as undifferentiated or poorly differentiated squamous cell carcinoma(SCC) arising from the midline structures of the body (lung, head, neck, bladder, etc.). Non-midline cases continue to be reported. Of these, 20%-25% of CUP cases are poorly differentiated and cannot be characterized histologically. A very high index of suspicion is required to rule out NC in cases of CUP, as it can commonly be misdiagnosed as poorly differentiated SCC. Approximately 5% of CUP cases are classified as squamous cell carcinoma, and by comparison, less than 200 cases of NC have been reported in the literature worldwide as of 2021[3].
NC is characterized by a chromosomal translocation that results in a NUT-fusion oncoprotein, the presence of which often is readily identified via nuclear immunohistochemical (IHC) staining positive for NUT, although McEvoy et al. recommend defining the nuclear protein in testis (NUTM1) fusion partner rather than relying on tumor morphology or immunohistochemical profile[4]. Diagnosis can be confirmed by molecular analysis techniques, including fluorescence in situ hybridization (FISH), reverse-transcription PCR, cytogenetics, and next-generation sequencing (NGS). It should be noted that while NGS testing can facilitate the diagnosis of NC, it can still result in a false negative[5].
NC was first described in two cases reported in 1991, with the most common chromosome translocation t(15;19)(q15;p13)[6]. This aggressive cancer has a median overall survival (OS) of 6.7 months and is likely the most aggressive solid tumor in humans[7,8]. Due to the rarity of this disease, a national registry was created in 2010 in order to raise awareness and collect clinical data on its characteristics and response to treatment[9]. Since that time, NC has been diagnosed more commonly, given increased awareness and better diagnostic capabilities.
The most common chromosomal rearrangement seen in NC is that of the NUTM1 gene on
Normally, NUT is a nuclear protein which binds histone acetyltransferase (HAT) and causes histone acetylation. In NC, the most common NUT fusion partners are part of bromodomain and extra-terminal (BET) family (i.e., BRD3 and BRD4). This family of proteins regulates transcription via epigenetics reading of acetylated histone lysine tail residues. BRD4 regulates transcription, cell growth, cell cycle, and chromatin structure driven by the BRD4 promoter. MYC and TP63 have been shown to be the key downstream target of this gene, which promotes uncontrolled cell growth. A study showed BRD4 was hyperphosphorylated in NC and cyclin-dependent kinase 9 (CDK9) was the potential kinase mediating this[12]. Therefore, by blocking BRD4 hyperphosphorylation with inhibitors, it was proposed that this may mitigate the proliferation of this deadly disease. NSD3 is an enzymatic protein involved in methylating histone lysines, which regulate gene expression and chromatin integrity[13].
CASE REPORT
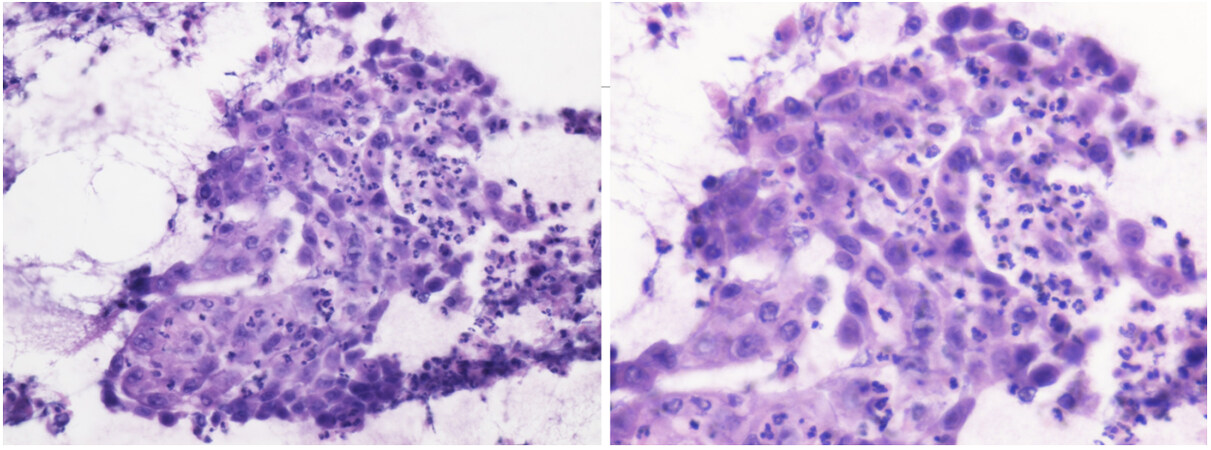
Our patient was a 27-year-old woman who presented initially with a thyroid mass to an outside hospital, which was diagnosed by biopsy as squamous cell carcinoma. The patient presented to our hospital for further management. She underwent a partial excisional biopsy of the thyroid mass, with final pathology showing muscular fibroconnective tissue with mixed inflammatory cells, giant cells, and focal necrosis [Figure 1]. No thyroid tissue was identified. Biopsy of the left strap muscle showed skeletal muscle with acute and chronic inflammation. Tumor cells were positive for p40, p53, epidermal growth factor receptor (EGFR), and a high Ki-67 labeling index (40%-70%). Scattered tumor cells were weakly positive for paired-box gene 8 (PAX-8) and rare tumor cells were positive for thyroid transcription factor 1 (TTF-1), which raised the possibility that there could be a component of anaplastic thyroid carcinoma. However, NGS testing eventually revealed the presence of a NUT fusion (NSD3-NUTM1), which is pathognomonic for NC.
Figure 1. H&E Stain: Left image (40×) and Right image (60×): Cytopathology specimen of left thyroid biopsy. Cell block showing detached atypical epithelial cells in loosely cohesive clusters with prominent nucleoli and wispy dense cytoplasm intermixed with marked neutrophils, suspicious for squamous cell carcinoma.
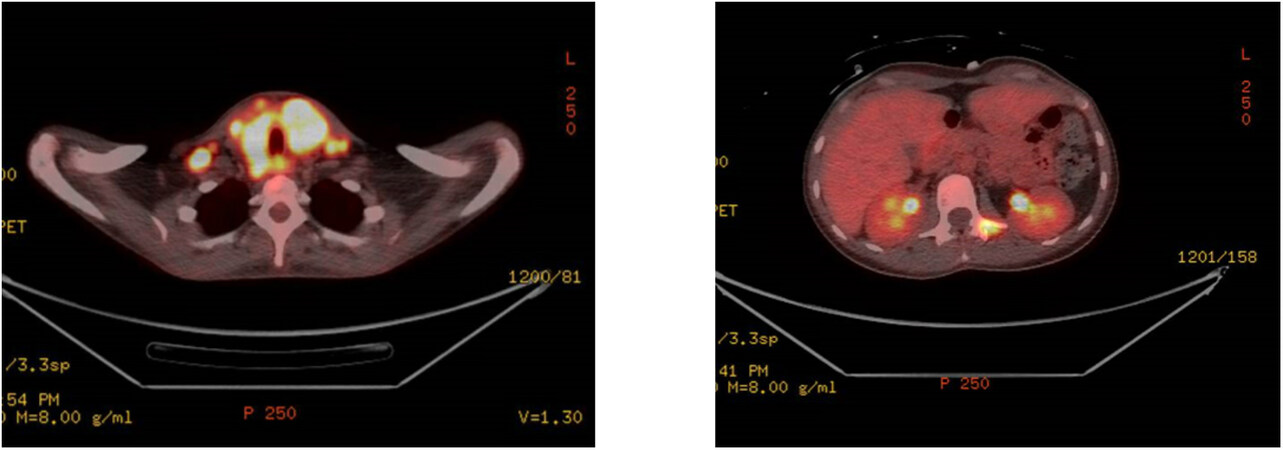
Despite a rapid work-up from presentation, she was found to already have metastatic disease at the time of diagnosis. Computerized tomography (CT) of the chest, abdomen, and pelvis showed a few scattered pulmonary nodules measuring up to 0.5 cm and a large thyroid mass with enlarged necrotic cervical lymph nodes (LN). CT of the neck showed a 2.6 cm × 2.7 cm × 4.1 cm hypoattenuating lesion of the left thyroid lobe, and bilateral pathologic cervical LNs (right measuring 3.1 cm × 2.2 cm level II/III and left 1.6 cm × 1.2 cm left level II/III). She was treated with carboplatin and paclitaxel weekly. Given high programmed death-ligand 1
Figure 2. PET/CT 64 days post-initial presentation: Left image (400×): FDG-avid diffuse enlargement of thyroid gland, compatible with known malignancy. FDG-acid bilateral retropharyngeal and extensive FDG-avid bilateral cervical lymphadenopathy. Right image
DISCUSSION
We conducted a literature review of the prognosis and treatment of NC. A PubMed for eligible studies from 1991 to 2021 published in the English language was performed using the following Medical Subject Headings (MeSH) terms: NUT midline carcinoma; NUT midline carcinoma thyroid; NUT midline carcinoma BET; NUT-midline carcinoma histone methyltransferase; NUT-midline immune checkpoint inhibitors; NUT-midline treatment. Results of the literature helped drive treatment for our patient.
When searching for “NUT midline carcinoma”, PubMed lists 202 results. Although most literature is described via case reports, Chau et al. performed a retrospective study of the NC registry[8]. This resulted in the largest retrospective study of 141 patients with NC. The median age at diagnosis in this cohort was 23.6 years old, and the median survival was 6.5 months (95%CI = 5.8 to 9.1 months) regardless of treatment[7]. The primary tumor location was found to be from the midline supradiaphragmatic structures, most often in the thorax (51% of patients), followed by other sites in the head/neck (41%), bone and soft tissue (6%), and others (1%). More than 60% of patients had one or more sites of metastases in this study. Survival was examined by anatomical site and genetics. Nonthoracic primary with BRD3 or NSD3-NUT median overall survival (mOS) was 36.5 months (95%CI = 12.5 to not reported months); nonthoracic primary BRD4-NUT had mOS 10 months (95%CI = 7 to 14.6 months), and thoracic primary had the worst prognosis with mOS 4.4 months (95%CI = 3.5 to 5.6 months). Additionally, complete surgical resection offered the biggest improvement in survival, especially for tumors less than 6 centimeters in size; chemotherapy and radiation treatment, unfortunately, are largely ineffective.
When adding the search term “thyroid,” four case reports were found. Agaimy et al. described a 42-year-old woman with thyroid sclerosing mucoepidermoid carcinoma with eosinophilia, a rare thyroid cancer, with concomitant NSD3-NUTM1 fusion diagnosed as NC[14]. Though our patient also harbored the same NSD3-NUTM1 fusion protein, her thyroid tumor’s morphology favored anaplastic thyroid carcinoma with squamous cell differentiation. Landa et al. described a 34-year-old woman who had a translocation involving NUTM1 and BRD4 fusion[15]. This patient underwent a total thyroidectomy and laryngopharyngectomy plus radiation and was alive 10 years after diagnosis, which is a clear outlier for survival[15]. A third recent case report described another NUTM1-BRD4 fusion in a 38-year-old male PD-L1 positive and TTF-1, P63, epithelial membrane antigen (EMA), and c-myc on immunohistochemistry[16]. Due to this patient being PD-L1 positive, similar to our patient, she was treated with chemotherapy followed by camrelizumab (a PD-1 inhibitor); however, she died 10 months after surgery. A fourth recently published case report describes a high-grade thyroid carcinoma with an NSD3-NUTM1 fusion, similar to our patient[17]. Interestingly, this patient also exhibited expression of PAX8 and TTF1.
When adding the search term “treatment,” 109 articles were found. No standard treatments have been established for NC; however, a multi-modality approach with surgery, systemic chemotherapy, and radiation therapy remains the backbone of current standard of care. Given the rarity of this disease, it is difficult to evaluate the efficacy of various treatment combinations. In a report utilizing the International NUT Midline Carcinoma Registry, 40 patients with head and neck involvement were analyzed; the biggest predictors for improved OS was initial surgery, with or without chemoradiation (chemoRT) or radiation
More recently, bromodomain inhibitors (BETis) and histone deacetylase inhibitors (HDACis) have emerged as promising agents in combination with chemotherapy, and there have been some benefits seen with immune checkpoint inhibitors and CDK9 inhibitors[19-21]. Epigenetics in cancer has shown that human cancer cells exploit both genetic and epigenetic abnormalities. HDACi has been proposed for the
When adding “BET” to the search term, 19 articles were found, the first one dating to 2012. It is hypothesized that BRD-NUT fusion proteins function as oncogenes by sequestering histone acetyltransferase activity, thus blocking differentiation[23]. BETis bind the acetyl-lysing pocket of bromodomains BD1 and BD2, thus being of possible use in treating tumors driven by BET fusion proteins (including BRDT, BRD2, BRD3, and BRD4) by blocking the binding of BRD-NUT proteins to chromatin and thus resulting in terminal differentiation of NC cells[24]. BETis are being extensively studied in both solid and liquid tumors. The acetyl-histone binding of BRD4-NUT bromodomains is required for the expression of MYC, and bromodomains are required to enrich the MYC promoter[25]. A phase I and II, open-label, dose-escalation study with the BETi molibresib was conducted and enrolled 65 total patients with various tumor types, including 19 patients with NC. The most frequent treatment-related adverse effects were thrombocytopenia (51%), gastrointestinal events including nausea, vomiting, diarrhea, dysgeusia
Birabresib was recently examined in a Phase 1b trial with 46 patients, 10 of whom had NC[27]. Six patients had partial response or stable disease. Stathis et al. described the use of Birabresib in four NC patients, describing dramatic and rapid response in two patients (OS of 18 and 19 months) and stable disease in a third patient[28]. All patients died of the disease but the mOS was longer than reported in the largest retrospective study, with OS of 6.7 months reported by Bauer et al. and 9.7 months reported by Chau and colleagues[7,18]. Adverse effects were found to limit the use of BETi, with the most common toxicities being diarrhea, nausea, decreased appetite, and thrombocytopenia. Given resistance to restoration of MYC expression, Liao et al. proposed combining BETi with cyclin-dependent kinase (CDK)4/6 inhibitors[29]. Clinical trials are currently being performed with CUDC-907, a combined histone deacetylase inhibitor (HDACi)/phosphatidylinositol 3-kinase inhibitor (PI3K inhibitor) for NC [NCT02307240].
Combining immune checkpoint inhibitors (ICIs) with BETi to treat NC is also under investigation[30]. A PubMed search resulted in 1 article by adding the term “immune checkpoint inhibitor”. Xie et al. performed a retrospective review of patients with primary pulmonary NC in the First Affiliated Hospital of Guangzhou Medical University between Jan 2015 and Dec 2018[31]. Seven patients were studied and five were treated with ICIs. One patient who received atezolizumab died after surgery due to severe postoperative complications (OS 1.5 months); another patient in the series received pembrolizumab following radiation therapy and had a good clinical response (OS 19.5 months), while another case treated with nivolumab and paclitaxel-albumin seemed to fail to benefit from treatment (OS of 3 months). Notably, another patient treated with cetuximab, docetaxel + platinum chemoRT, followed by pembrolizumab and oxaliplatin on the progression of disease had an OS of 26.7 months. The only patient alive at the time of follow-up (OS
No studies were found using the PubMed search for NUT midline carcinoma and histone methyltransferase as treatment.
Given that our patient did not have a BRD4-NUT fusion, she was not a candidate for enrolling in a BETi clinical trial. Instead, our patient was treated with surgical removal of the primary tumor, radiation therapy, and systemic treatment with weekly carboplatin and paclitaxel and pembrolizumab. In the literature, thus far, there have been no proposed novel therapeutics that specifically target the fusion protein NSD3-NUT, likely because this gene combination is less common than BRD4 or BRD3 fusion protein. However, we would like to propose that additional research should be performed on the addition of HMTis as part of the therapy for this specific gene mutation. Recently, the HMTi tazemetostat has been approved for metastatic or locally advanced epithelioid sarcoma. Many other HMTis are in development.
CONCLUSION
NUT midline carcinoma is a rare, devastating, and universally fatal disease within 3 years of diagnosis, with median overall survival ranging between 6.7 to 9.7 months[4,11]. Chemotherapy, surgery, and radiotherapy improve survival without providing curative results. By introducing additional agents such as ICIs and BETis, disease-free survival has been promising. We propose that for patients with NRD3-NUT translocation, exploring the use of HMTis as adjunctive therapy may provide additional survival benefits. We also highlight a case of NC mimicking anaplastic thyroid carcinoma with squamous cell differentiation, where NGS helped expedite an accurate diagnosis. We further propose that diagnostic evaluation of young patients with CUP and squamous features include NC in the differential diagnosis, given the rapidly progressive nature of this disease.
DECLARATIONS
Authors’ contributionsWas the main author who wrote the initial draft (Abstract, Introduction, Case Report, Discussion, and Conclusion) of the manuscript, performed the literature review and edited the manuscript: Neumann M
Reviewed the manuscript and edited the manuscript: Hines A
Provided the figures and their captions: Chang Q
Reviewed each draft of the manuscript, coordinated acquiring the figures, and edited the manuscript in detail: Seetharamu N
Reviewed the manuscript and edited the manuscript in detail: Lopez C
Availability of data and materialsData regarding the case report can be found on Northwell’s EMR. Data regarding the literature review is referenced in the manuscript as footnotes and can be found on PubMed.
Financial support and sponsorshipNone.
Conflicts of interestAll authors declared that there are no conflicts of interes.
Ethical approval and consent to participateNot applicable.
Consent for publicationConsent for publication has been obtained from the next of kin of the participant.
Copyright© The Author(s) 2022.
REFERENCES
2. Available from: https://www.cancer.org/content/dam/CRC/PDF/Public/8589.00.pdf [Last accessed on 24 Jun 2022].
3. Jiang J, Ren Y, Xu C, Lin X. NUT midline carcinoma as a primary lung tumor treated with anlotinib combined with palliative radiotherapy: a case report. Diagn Pathol 2022;17:4.
4. McEvoy CR, Fox SB, Prall OWJ. Emerging entities in NUTM1-rearranged neoplasms. Genes Chromosomes Cancer 2020;59:375-85.
5. Kim YH, Song Y, Kim JK, et al. False-negative errors in next-generation sequencing contribute substantially to inconsistency of mutation databases. PLoS One 2019;14:e0222535.
6. Kubonishi I, Takehara N, Iwata J, et al. Novel t(15;19) chromosome abnormality in a thymic carcinoma. Cancer Res 1991;51:3327-8.
7. Bauer DE, Mitchell CM, Strait KM, et al. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res 2012;18:5773-9.
8. Chau NG, Ma C, Danga K, et al. An anatomical site and genetic-based prognostic model for patients with nuclear protein in testis (NUT) midline carcinoma: analysis of 124 patients. JNCI Cancer Spectr 2020;4:pkz094.
9. Available from: https://nmcregistry.org/ [Last accessed on 24 Jun 2022].
10. Huang QW, He LJ, Zheng S, Liu T, Peng BN. An overview of molecular mechanism, clinicopathological factors, and treatment in nut carcinoma. Biomed Res Int 2019;2019:1018439.
11. Prall OWJ, Thio N, Yerneni S, Kumar B, McEvoy CR. A NUT carcinoma lacking squamous differentiation and expressing TTF1. Pathology 2021;53:663-6.
12. Brägelmann J, Dammert MA, Dietlein F, et al. Systematic kinase inhibitor profiling identifies CDK9 as a synthetic lethal target in NUT midline carcinoma. Cell Rep 2017;20:2833-45.
13. Han X, Piao L, Zhuang Q, Yuan X, Liu Z, He X. The role of histone lysine methyltransferase NSD3 in cancer. Onco Targets Ther 2018;11:3847-52.
14. Agaimy A, Tögel L, Stoehr R, et al. NSD3-NUTM1-rearranged carcinoma of the median neck/thyroid bed developing after recent thyroidectomy for sclerosing mucoepidermoid carcinoma with eosinophilia: report of an extraordinary case. Virchows Arch 2021;479:1095-9.
15. Landa I, Ibrahimpasic T, Boucai L, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest 2016;126:1052-66.
16. Zhou J, Duan M, Jiao Q, et al. Primary thyroid NUT carcinoma with high PD-L1 expression and novel massive. IGKV ;11:778296.
17. Allison DB, Rueckert J, Cornea V, Lee CY, Dueber J, Bocklage T. Thyroid carcinoma with NSD3::NUTM1 fusion: a case with thyrocyte differentiation and colloid production. Endocr Pathol 2022;33:315-26.
18. Chau NG, Hurwitz S, Mitchell CM, et al. Intensive treatment and survival outcomes in NUT midline carcinoma of the head and neck. Cancer 2016;122:3632-40.
19. Lim SL, Xu L, Han BC, Shyamsunder P, Chng WJ, Koeffler HP. Multiple myeloma: Combination therapy of BET proteolysis targeting chimeric molecule with CDK9 inhibitor. PLoS One 2020;15:e0232068.
20. Jung M, Kim S, Lee JK, et al. Clinicopathological and preclinical findings of NUT carcinoma: a multicenter study. Oncologist 2019;24:e740-8.
21. Zhao C, Dong H, Xu Q, Zhang Y. Histone deacetylase (HDAC) inhibitors in cancer: a patent review (2017-present). Expert Opin Ther Pat 2020;30:263-74.
22. Shiota H, Alekseyenko AA, Wang ZA, et al. Chemical screen identifies diverse and novel histone deacetylase inhibitors as repressors of NUT function: implications for NUT carcinoma pathogenesis and treatment. Mol Cancer Res 2021;19:1818-30.
24. Napolitano M, Venturelli M, Molinaro E, Toss A. NUT midline carcinoma of the head and neck: current perspectives. Onco Targets Ther 2019;12:3235-44.
25. Grayson AR, Walsh EM, Cameron MJ, et al. MYC, a downstream target of BRD-NUT, is necessary and sufficient for the blockade of differentiation in NUT midline carcinoma. Oncogene 2013;33:1736-42.
26. Piha-Paul SA, Hann CL, French CA, et al. Phase 1 study of molibresib (GSK525762), a bromodomain and extra-terminal domain protein inhibitor, in NUT carcinoma and other solid tumors. JNCI Cancer Spectr 2020;4:pkz093.
27. Lewin J, Soria JC, Stathis A, et al. Phase Ib trial with birabresib, a small-molecule inhibitor of bromodomain and extraterminal proteins, in patients with selected advanced solid tumors. J Clin Oncol 2018;36:3007-14.
28. Stathis A, Zucca E, Bekradda M, et al. Clinical response of carcinomas harboring the BRD4-NUT oncoprotein to the targeted bromodomain inhibitor OTX015/MK-8628. Cancer Discov 2016;6:492-500.
29. Liao S, Maertens O, Cichowski K, Elledge SJ. Genetic modifiers of the BRD4-NUT dependency of NUT midline carcinoma uncovers a synergism between BETis and CDK4/6is. Genes Dev 2018;32:1188-200.
30. Hogg SJ, Vervoort SJ, Deswal S, et al. BET-bromodomain inhibitors engage the host immune system and regulate expression of the immune checkpoint ligand PD-L1. Cell Rep 2017;18:2162-74.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Neumann M, Hines A, Chang Q, Seetharamu N, Lopez CA. NUT midline carcinoma presenting initially as thyroid cancer: a case report and review of treatment. J Transl Genet Genom 2022;6:353-60. http://dx.doi.org/10.20517/jtgg.2022.06
AMA Style
Neumann M, Hines A, Chang Q, Seetharamu N, Lopez CA. NUT midline carcinoma presenting initially as thyroid cancer: a case report and review of treatment. Journal of Translational Genetics and Genomics. 2022; 6(3): 353-60. http://dx.doi.org/10.20517/jtgg.2022.06
Chicago/Turabian Style
Neumann, Melissa, Adam Hines, Qing Chang, Nagashree Seetharamu, Carlos A. Lopez. 2022. "NUT midline carcinoma presenting initially as thyroid cancer: a case report and review of treatment" Journal of Translational Genetics and Genomics. 6, no.3: 353-60. http://dx.doi.org/10.20517/jtgg.2022.06
ACS Style
Neumann, M.; Hines A.; Chang Q.; Seetharamu N.; Lopez CA. NUT midline carcinoma presenting initially as thyroid cancer: a case report and review of treatment. J. Transl. Genet. Genom. 2022, 6, 353-60. http://dx.doi.org/10.20517/jtgg.2022.06
About This Article
Special Issue
Copyright
Data & Comments
Data
 Cite This Article 10 clicks
Cite This Article 10 clicks












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.