The importance of genetics in an advanced integrative model of autism spectrum disorder
Abstract
Genes have long been considered to cause autism spectrum disorder (ASD). However, data obtained over the last 10 years indicate that the true role of genetics in ASD and the cost-benefit ratio of genetic testing following an ASD diagnosis warrant further investigation. ASD is heterogeneous with high individual complexity, and new findings related to systemic alterations in ASD (in addition to genetics) should be considered when attempting to optimize patient health. However, for some people with ASD and their families, genetic testing can identify genetic mutations or chromosomic alterations. This review mainly considers recent research (the last 5 years) on the role of genetic factors in ASD and the importance of genetic testing in a new Advanced Integrative Model of ASD.
Keywords
INTRODUCTION
Studies spanning the past 50 years have indicated the heterogeneous nature of autism spectrum disorder (ASD), not only in terms of clinical presentation but also the underlying etiology[1]. Although increasing numbers of studies with large sample sizes have enabled researchers to identify common genetic factors associated with autism, the genetic predisposition for ASD may be different in each individual[2]. As discussed by Hoang et al., genetic heterogeneity seems to be a common feature of clinical sequencing data. Thus, although a broad range of evidence has indicated an association between genes and ASD, variations in gene expression and non-penetration are today accepted as widespread[1].
Nowadays, many commercially available gene panels are offered. However, other risk factors have been associated with ASD aside from genetic susceptibility (such as prenatal diet and prematurity)[3].
Nowadays, gene lists from autism gene panels have been found to be insufficient for ASD diagnosis, as further evidence is needed[5]. As clearly discussed in a recent paper, if the application of a targeted gene panel to an affected individual’s genome is negative for pathogenic variation, one cannot conclude that a contributive variant is not present[6]. Under these circumstances, other factors should be considered in addition to genetics, such as selected metals, iodine, vitamins, folate, and biotin[7]. Within the framework of epigenetics, brain-body homeostasis must be taken into consideration. Beyond genetics, epigenetics, and transcriptomics within and outside the brain, physiology and systems biology should be taken into account in understanding ASD.
Given the complexity of ASD, as well as its heterogeneity, an overall approach must include environmental, nutritional, and immune as well as genetic factors. Consequently, ASD diagnoses based only on the results of commercial gene panels might have limited clinical accuracy, unless there is clear evidence of a genetic abnormality (e.g. suspected Fragile-X syndrome, FXS). This should be considered when developing procedures for primary screening, particularly in developing countries in which gene panel diagnoses might be cost-prohibitive for the majority of the population.
Likewise, and especially considering those in developing countries with limited access to healthcare services, healthcare providers should assess the cost-benefit ratio of performing genetic tests when there is no syndromic phenotype for ASD. The main problem lies in the cost of genetic studies and the fact that the findings regarding non-pathogenic mutations do not offer a therapeutic perspective. Indeed, identifying a mutation outside a specific clinical context does not provide tools for treatment and can increase the costs for the family or medical services providers. It can also seriously affect the will of the family and the therapeutic and medical team regarding the opportunities for the patient. The detection of a mutation does not imply pathogeny or an automatic correlation with ASD. However, the concomitant medical problems (CMP) to ASD diagnosis have individual importance, and they should be correctly evaluated, diagnosed, and treated.
The recent literature shows strong evidence of autoimmunity linked with ASD in individuals with FXS. Lisik et al. reported that a subgroup of FXS patients (43.48%) tested positive for serum anti-neuronal antibodies[8]. Thus, autoimmune factors may cause or contribute to autistic symptoms in that group.
Song et al. examined the administration of antioxidants to delay the onset of FXTAS, with the goal of decreasing morbidity and treating the concomitant mitochondrial dysfunction[13]. In 2014, Valenti et al. proposed that mitochondria and radical oxygen species play a role in the pathogenesis of various known genetic disorders (such as Down’s, RTT, and FXS)[14]. The authors reviewed novel therapeutic approaches involving the activation of mitochondrial function and the reduction of oxidative stress[14]. In 2019, Müller considered the presence of altered mitochondria and redox status in individuals with RTT syndrome, and evaluated the use of medications such as antioxidants and scavengers to stabilize these issues[15].
THE ROLE OF GENETICS IN DIFFERENT MODELS OF ASD
Genetic models
In 2022, a study by Carlsson et al. examined a diverse range of models for the genetic and environmental mechanisms of ASD. The authors stated, “Although highly heritable, environment also contributes to the etiology of ASD”[16]. According to the cumulative stress hypothesis and the three-hit concept, vulnerability for a given condition is enhanced if adversities are accumulated during the early stages of life. In this framework, genetic predisposition and the later-life environment are considered as the first and third hits, respectively[16]. In the multi-dimensional model of ASD, underlying genetic and environmental factors are assumed to continuously affect the risk of developing ASD. In 2021, Torres emphasized the importance of considering the brain-body connection and the contribution of the peripheral nervous system to mental health[17]. The author and her group combined the datasets from genes associated with mental illnesses with well-known neurological disorders and with illnesses that are not directly associated with the nervous systems and with manifestations of acquired Post-Traumatic Stress Syndrome Disorder (PTSD). They included autism, schizophrenia and general, bipolar and unipolar depression. Among neurological conditions, ataxias (e.g., cerebellar, spinocerebellar, progressive, and gait) and Parkinson’s disease were taken into account. Among non-neurological disorders, they considered colon cancer, breast cancer, diabetes, congenital heart disease, hematologic neoplasm, and several autoimmune disorders. The selection was done to include neuromotor aspects, related to links between the brain and the peripheral nervous system (mainly the autonomic nervous system). Torres reported as surprising the finding of contributions of peripheral structures and organs to mental illness. She included in her work: “Tissues of the autonomic nervous systems were maximally impacted by the removal of the genes associated with these mental illnesses, as was the muscle-skeletal tissue among the top-ranked illnesses. Tissues associated with subcortical brain regions necessary for motor control, learning, adaptation, and coordination (basal ganglia and striatum) were highly impacted by the removal of the genes in both mental illnesses and neurological disorders, along with those tissues important for memory (hippocampus), emotion (amygdala) and regulation (hypothalamus)”.
Major neuropsychiatric conditions that are behaviorally defined using the Diagnostic and Statistical Manual of Mental disorders (DSM) criteria have strong convergence with well-known neurological conditions (e.g., ataxias and Parkinson’s disease), but less overlap with non-neurological conditions. Psychiatric diagnoses affect both tissues involved in the central control and peripheral (heart and muscle-skeletal) nervous systems. The results of Torres emphasize the importance of considering both the brain-body connection and the contributions of the peripheral nervous systems to mental health[17]. These relationships are not taken into account in the classic approach of gene causation in ASD, even when the complexity has been demonstrated to be huge. CMP outside the brain (cited above), neurological issues (epilepsy/convulsions, sleep issues, movement disorders) and psychiatric diagnoses co-occur many times in the same person with the ASD diagnosis. A recent manuscript in the field of pediatrics (2022) cited a known problem in genetic studies, that is, that heritability estimates are primarily derived in such a way that they may overrate the role of genes. Moreover, in most cases, gene-only (G) and genetic (G)* shared environmental influences (E) (cited as GxE) are considered to be genetic[18]. GxE involves the interaction between the genotype and the environment. The problem of identifying genetic contributions and neglecting the possibility that genes act in concert with environmental agents is a general one, and occurs even in recent studies. Indeed, Volk et al. stated that it “is highly likely that the interplay of gene variants and environmental factors contributes to a substantial proportion of autism”[18]. A systematic review reported wide-ranging heterogeneity in the prevalence of co-occurrent medical problems with ASD. They found that participants with ASD also had attention deficit hyperactivity disorder (ADHD; to 86.00%), anxiety (up to 82.20%), depressive disorders (up to 74.80%), epilepsy (2.80%-77.50%), intellectual disability (ID; up to 91.70%), sleep disorders
In a systematic review from 2021, Wei et al. reported that more than 200 susceptibility genes had been correlated with autism[22]. For almost every chromosome, cytogenic abnormalities have been reported. According to the authors, candidate genes related to ASD might exist in nearly every chromosome[22]. However, these findings, along with those of other epigenetic studies at the brain level only, do not consider how translational actions can improve the quality of life and outcomes in people with ASD. Indeed, the influence of outside-the-brain factors deserves more attention, even though genes and genetic pathways may be essential in unraveling the pathogenesis of ASD from a mechanistic point of view. For instance, in a study of 1031 genes, David et al. reported that only 262 were directly associated with ASD, whereas the others were related to other comorbid disorders[23]. Furthermore, Díaz-Beltran et al. used a two-fold system biology approach to propose a multi-comorbidity subtype of ASD[24]. Finally, Klein et al. used a topic modeling approach and concluded that a better understanding of the underlying pathogenetic mechanisms involved in medical issues co-occurring with ASD may have clinical implications in the comprehensive assessment and management of these patients[25].
Advanced Integrative Model
The Advanced Integrative Model (AIM) considers the ASD diagnosis as the name of an emerging symptomatology. The underlying condition at the brain level may be thought of as a chronic, dynamic, and systemic form of encephalopathy, a priori with respect to an ASD diagnosis, which is potentially reversible[26]. The reversibility would be closely related to the genetic susceptibility or risk, the amount and severity of medical problems outside the brain that affect the brain (CMP), and the approach for addressing CMP. The lack of treatment for CMP could adversely affect ASD symptoms trajectory and development. CMP are also influenced by genetic susceptibility, such as risk related to genetic polymorphisms, genetic mutations, and chromosomal alterations.
In a 16-year pediatric cohort study in Canada (1993-2010), Cawthorpe assessed the quantity of CMP in individuals with ASD. CMP were diagnosed by a physician according to the International Classification of Disease version 9 (ICD-9). The analysis included 111 female and 609 male participants with ASD. According to this study, 28 ICD disorders were found before and 95 ICD disorders after an ASD diagnosis in female participants, whereas 38 ICD disorders preceded and 234 ICD disorders proceeded ASD diagnosis in male participants[27].
The chronic, dynamic, systemic encephalopathy framework
In this framework, genetic polymorphisms related to the immune system, mitochondrial dysfunction, autoimmune susceptibility and genes expressed in tissues beyond the brain are important in terms of ASD risk. Over the last decades, many behaviors have been assigned to ASD. However, some of these behaviors may be the emerging symptomatology of an encephalopathy related to underlying medical problems outside the brain, which can affect the brain through barrier permeabilities and other mechanisms. Accordingly, gut and blood-brain barrier (BBB) permeabilities in ASD are receiving increasing attention from researchers. The gut-brain-microbiota axis, gut-brain vagal connection, and the complex relationship between behavior and immune system function is increasingly being taken into account. Allergies and food intolerances are considered to have a large effect on the symptoms of ASD. In this sense, the evaluation, detection, and treatment of CMP are extremely important, and healthcare practitioners should be aware of the importance of medical problems such as diarrhea, constipation, vitamin deficiencies (D, B complex,
Regression is defined as losing developmental skill(s) or ability/ies (among them language and social engagement) that a child once had. Regression of up to 88% was reported in prospective studies about infants who were at risk for ASD[28]. Mitochondrial dysfunction has been linked to regression[29] as well as autoimmunity in ASD[30]. Redox alterations and mitochondrial dysfunction have been considered to play a role in the presentation of RTT[15,31].
The static encephalopathy framework
Genetic testing may be critical in understanding individual complexity in several medical problems. For example, epilepsy, movement disorders, and developmental delay are associated with a glucose transporter type 1 (GLUT 1) deficiency. This has also been associated with impaired glucose transport across the BBB in individuals with ASD[32].
The QT interval is the lapse from the beginning of the Q wave to the end of the T wave during ventricular depolarization and repolarization in the heart. In long QT syndrome (LQTS), the heart takes more time to return to the baseline between beats. Timothy syndrome is a congenital form of LQTS with syndactyly and developmental delay. The prevalence of ASD in individuals with Timothy syndrome is nearly 70% of all cases.
Kreiman and Boles assessed five children with ASD and genetic mutations in whom genetic testing provoked certain therapeutic actions and finally an improvement in well-being[32]. In three of the five cases (TNF-Receptor Associated Protein 1 gene [TRAP1], choline acetyltransferase [CHAT], and Solute Carrier Family 6 Member 8 [SLC6A8] mutations in 3 different patients), important positive changes in the
Despite the many genetic syndromes described, alterations in the chromosome microarray (CMA) may only occur in 9%-10% of people with ASD, whereas that in the whole genome, such as in Exome whole sequencing (EWS), may only occur in up to 21% of people with ASD. Indeed, a positive correlation does not imply causation, but merely a contribution, which may be shared with other contributors.
Stafford et al. examined several cases of genetic syndromes characterized by the specific and acute presentation of symptoms and/or dysmorphisms, in which genetic confirmation was necessary to determine appropriate medical interventions[33]. For these medical genetic issues, genetic testing is undoubtedly warranted. However, instead of recommending genetic testing only for this subgroup, the Genetic Model proposes genetic testing for all people diagnosed with ASD. This approach might be prohibitive, especially for low-income countries.
In a systematic review and meta-analyses from 2015, estimates of ASD range from 11% for 22q11.2 deletion syndrome to 61% in females with RTT[34]. For all syndromes, odds ratios showed that people with ASD have a higher risk of genetic abnormalities than the general population. Several studies have examined the overlapping genetic traits in individuals with epilepsy/seizures and ASD and in those with psychiatric diagnoses and ASD[35]. Frye et al. examined the biological abnormalities shared by ASD and epilepsy, and found abnormalities in minicolumn architecture and GABA neurotransmission[36]. Medical abnormalities associated with both ASD and epilepsy include genetic syndromes, metabolic disorders, mineral and vitamin alterations, heavy metal bioaccumulation, and immune dysfunction[36]. Treatment-resistant epilepsy was more prevalent in children diagnosed with ASD (i.e., they had a mitochondrial dysfunction or disorder and alterations in cerebral folate transport) compared with those without an ASD diagnosis[37].
Recently, the heterogeneity of ASD was confirmed in a large study by Kuo et al.[38]. However, genetic ASD research continues as if the role of genes in ASD is causal and not contributive[39]. Indeed, MTHFR C667T is the only variant that has been found to indicate genetic vulnerability for ASD diagnosis. More than 1000 genes involved in ASD were reported by Doi et al., who suggested that ASD is a neurodevelopmental disorder (NDD) related not only to genetic but also environmental factors[40]. This approach is distinct from that in previous manuscripts about genes and ASD. A new framework and research avenues are needed to increase the quality of life of individuals diagnosed with ASD. Multiple studies about CMP in people with ASD have supported the need for new models[26,41-44], especially considering the high rate of early death in people with ASD[42] among other important topics. Finally, a complete review[45] indicated that the evidence accumulated during the last 20 years (but mainly the last 5 years) demonstrates that the genetic commonalities among individuals with ASD are only associations and have no direct causation link, i.e., they are related to ASD risk and vulnerability but are not deterministic.
HOW THE ENVIRONMENT AFFECTS VULNERABLE NEWBORNS: THE ROLE OF CMP IN TODDLERS AND INFANTS WITH ASD
Over the last 5 years, many articles have been published in high-quality journals with new evidence supporting the formation of models beyond the genetic model (GM) and neurodiversity model (NM). One example is Panisi et al., who established that the epidemiological and clinical findings in ASD cannot be explained by the GM[46]. They supported the development of a “more fluid conception, integrating genetics, environment, and epigenetics as a whole”. Indeed, a complex interplay of immune activation, dysbiosis, and mitochondrial impairment/oxidative stress could affect neurodevelopment during pregnancy and throughout life, leading to a concept of whole-body dysfunction in ASD. The authors proposed a “multidisciplinary approach and interdisciplinary sharing of knowledge”.
Special issues of the Journal of Personalized Medicine about ASD in 2021[47] and 2022[48] include a plethora of information about these topics from different research groups around the world. Recent manuscripts have correlated ASD and autoimmune issues as autoimmune encephalitis[49] and presented sociological aspects and clinical challenges for treating CMP in people diagnosed with ASD[50]. There is a clear need for models that take into account the complexity and heterogeneity of ASD and introduce new meaning to the role of CMP. Even authors who support conventional models are aware of the need for a revised approach[51]. For instance, Chen et al.[51] concluded that, “there is a need to pause, rethink, and discuss an intervention research agenda that better addresses the developmental and dynamic nature of Autism, and to adopt methodological approaches that support the shift of focus from macro- to micro-level change, as well as from static to dynamic prediction of change”. Recent reviews have emphasized the reemerging roles of the immune system in ASD[52]. Even when these aspects appear in the open literature, they are not frequently analyzed in terms of clinical practice. Instead, research is frequently focused on searching for biomarker(s) linked to certain genetic finding(s) to define epigenetics and then diagnosis and treatment. The research focused on biomarkers derived from genetic studies at the brain only ignores the possibility that the CMP, outside and at the brain, are the result of multiple genetic vulnerabilities interacting in parallel and in sequence with the environment (water, air, soil) and the exposome (food, infections, medications during the first 3 years of life, anesthesia, xenobiotics, and other biological stress sources) through epigenetics. Therefore, the research does not focus on enhancing the daily life of the person with ASD and the way in which his/her family addresses multiple CMP, their consequences, and concomitant neurological and psychiatric medical problems as a whole.
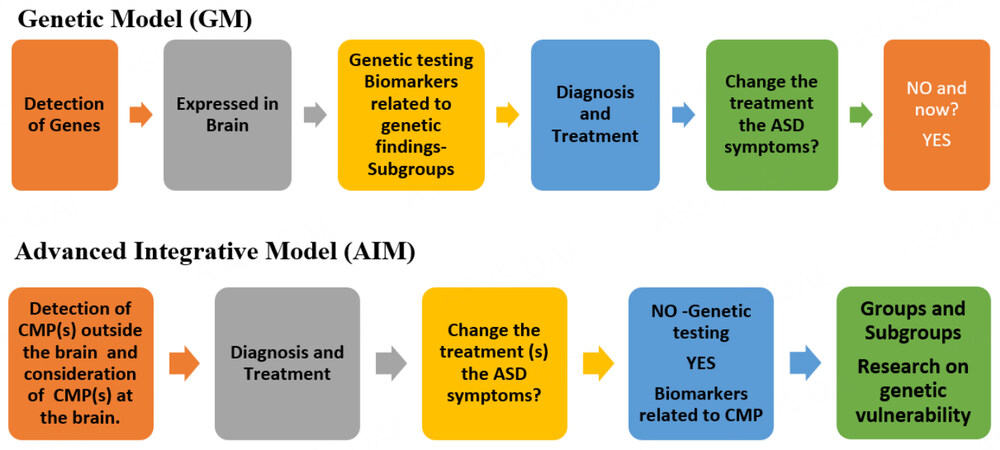
Scheme 1 shows the different approaches in the GM and AIM for the detection of Groups/Subgroups in people with ASD. Today, research about biomarkers in the GM is in the early stages because few validation studies have been conducted, and the available studies did not consider biomarkers using adequate comparison groups[53]. After almost 50 years of continuous research, we are at the beginning of the third of six steps, as shown in Scheme 1.
Scheme 1. Different approaches in the Genetic Model (GM) and Advanced Integrative Model (AIM) with respect to CMP, biomarkers, and genetic testing.
In the AIM, the CMP at the whole body level are first considered, properly diagnosed, and treated (including but not limited to GI issues, allergies or sensitivities to food composition, infections, dysbiosis, nutritional, metabolic, mitochondrial, biochemical, endocrinological, immune, and autoimmune issues, and oxidative stress). The responses to treatment of the CMP would produce data regarding ASD groups and subgroups and potentially reveal groups of biomarkers within subgroups. Neurological and psychiatric medical issues are considered in the AIM in terms of individual presentation and severity. With this stratification, research about genetic vulnerabilities (mutations and polymorphisms) is guaranteed and genetic testing has an important place. However, even if the treatment of a CMP via the AIM does not change ASD symptoms, the treatment is likely to improve the quality of life of the patient. In contrast, in the GM, there is no clinical exploration regarding medical issues outside the brain, and CMP may remain undetected, undiagnosed, and untreated.
THE ROLE OF GENES AND GENETIC TESTING IN THE AIM FOR ASD
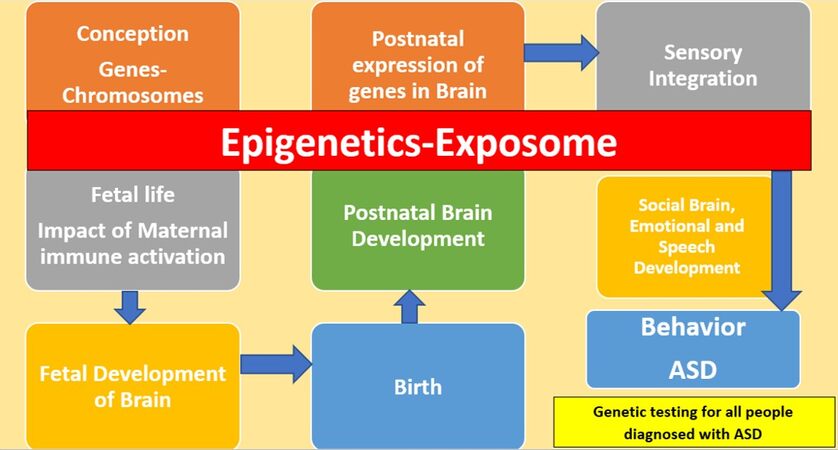
The GM, as presented in Figure 1, relates behavior to the effect of genes from conception to fetal prenatal brain development in a deterministic manner. Even when epigenetics are considered, the focus of the field is largely the prenatal life and the brain. In this model, the body, the system biology, and the postnatal development of the whole body are not discussed. The CMP outside the brain in people with ASD are dismissed as irrelevant with respect to the ASD core symptoms, and may also be considered compensation effects related to the effect of genetic vulnerability. Many previous studies have demonstrated the need to profoundly revise this application of the GM. The push to continue in this line of research has generated profound divisions among the parents of people diagnosed with more severe ASD, and high need of support, and people diagnosed with presentations of ASD that require less support.
Figure 1. The GM of ASD from conception to behavior with genetic testing for all people diagnosed with ASD. ASD: Autism spectrum disorder.
The focus on genes as causation in ASD could diminish the value of genetic testing for those cases where it is useful and even life-changing (i.e. mitochondrial disorders). Active research regarding the biomarkers of ASD is focused on genetic findings, brain mechanisms[54,55], and proteomics[56]. The final goal is to detect subgroups in people diagnosed with ASD using biomarkers, and to use this information to guide treatment.
Although this research has some important aspects, there are other approaches and mechanisms that are related to the integration of genetics-epigenetics and the effect of the exposome on whole-body function, which are centered on the CMP found in different ASD studies. For instance, a recent manuscript presented a mechanism for the generation of ASD considering the whole body, the gut-brain axis, and the brain and gut permeabilities[57].
Our previous manuscript presented a way to group individuals with ASD according to the individual responses to treatments for CMP[26]. Three main groups can be identified depending on the response (positive or null) to treatments for CMP. The first group overcomes the ASD diagnosis following the treatment of CMP, the second group exhibits changes in ASD symptoms, i.e., a reduction in support needs in response to the treatment of CMP, and the third group shows no changes in ASD symptoms.
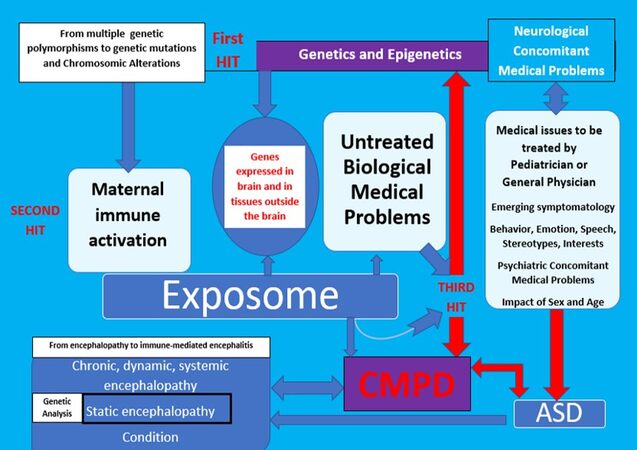
Figure 2 shows how genetic testing should be promoted in cases where static encephalopathy is suspected. This is mainly when the presentation of symptoms is acute, from birth, or the diagnostic criteria for genetic syndromes are met. When properly diagnosed CMP are present, treatment should be given opportunely and rigorously. In the context of the AIM, the treatment of emerging CMP symptomatology can provide clues about the dynamic and potentially reversible nature of encephalopathy. Because of systemic involvement, system biology must be considered. In this sense, genetic susceptibility, on a continuum from very mild to very strong, should be analyzed not only at the level of impact in neural synapses and receptors but also in tissues outside the brain. Furthermore, genetic vulnerability should be analyzed in terms of the whole-body impact on the development of CMP, including immune receptors, immune responses to infections, immune components, gut permeability, and known genetic links to CMP such as autoimmunity and dysbiosis. The risk of ASD should be analyzed in terms of risks of CMP, including barrier permeabilities, autoimmunity, inflammation, and neuroinflammation with the potential to generate an encephalopathic state. Figure 2 shows that the whole body must be considered and that the three-hit model is useful for understanding the development of a potential encephalopathy as the underlying nature of emerging ASD symptomatology at prenatal, neo-peri, and postnatal periods, beyond genetics. By considering the correlations between whole-body inflammation and CMP outside the brain with other neurological and psychiatric issues, this new model could bring together the fields of genetics, neurology, psychiatry, and pediatrics in the context of personalized, translational medicine. CMP outside the brain are considered key after ASD diagnosis in the AIM because they may contribute to the encephalopathy. Considering the sex and age of the patient, we expect that application of the AIM will produce high-quality evidence regarding the mechanisms underlying many psychiatric and neurological problems from childhood to adulthood. To achieve this goal, genetic information must be analyzed in a new way with respect to ASD. The role of genetics in an AIM is different, as the approach is a collaborative and transdisciplinary way to personalize whole-body medicine to optimize psychological, emotional, cognitive, and social health.
Figure 2. The AIM: role of the CMP and analysis of the response to treatments of CMP prior to genetic testing. ASD: Autism spectrum disorde; CMPD: concomitant medical problems to diagnosis.
In the framework of AIM, children with an ASD diagnosis and strongly suspected syndromic autism would qualify for CMA and Exome sequencing (ES). The goals in these cases are to know the causes, not to diagnose, and if secondary findings are found, to complete the genetic evaluation. Recent results from Ohashi et al. confirmed that only 10% of people with non-syndromic autism had findings of genes related to ASD in the ES[58] as well as other genetic reports[33]. The authors were clear that the identification of gene variants was not easy to interpret or apply in clinical practice.
CMP outside the brain and those related to known vulnerabilities in ASD should be evaluated, diagnosed, and treated according to the protocols for syndromic and non-syndromic autism. Even in syndromic autism (ASD with XFS[59] or Down’s syndrome[60]), there are many CMP that should be diagnosed and treated carefully.
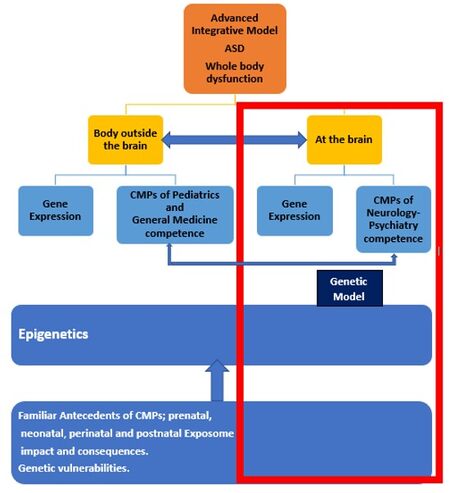
Figure 3 shows the approach of the AIM and how the GM is included. The brain and body interact through different pathways and mechanisms, and CMP outside the brain may influence neurological and psychiatric CMP in people with ASD[47,48]. The findings of genetic testing should not be considered as confirmation that a patient’s overall presentation is solely related to genetics, especially given the exhaustive clinical studies on CMP that are needed. Finally, genetic testing (CMA, Exome sequencing (ES)-EWS) may be useful when applied to syndromic autism, for both the patient and his/her parents and caregivers. It enables clarification of the overall situation and often provides clues regarding other medical issues.
CONCLUSION
*Whereas the GM assumes a linear relationship between a behavior and the effect of genes from conception to fetal prenatal brain development, the AIM of ASD takes a transdisciplinary approach in which the whole body must be considered. The AIM considers not only genetics but also epigenetics and neurological, psychiatric, pediatric medical, environmental, exposome, emotional, and social factors via a
*Genetic tests should be considered as a part of a complex frame in which their contributions are acknowledged for particular medical genetic issues (such as Cornelia de Lange, DiGeorge, and FXS). This is especially the case in children with developmental issues since birth or the first months of life or those with clear symptoms of chromosomic differences (Down’s syndrome) and an ASD diagnosis. In individuals with a genetic syndrome, regression is sometimes found with the subsequent diagnosis of ASD. Genetic testing is important in individuals with clear signo-symptomatology of genetic diseases or vulnerabilities with different complexity/presentation and evolution (such as polymorphisms in the MTHFR gene or mitochondrial disorders). Based on the response to treatments of CMP in people with ASD, anamnesis, dysmorphisms, and trajectory, genetic testing focused on specific subgroups could provide clues regarding pathogenesis and etiology.
*Regression (as loss of social abilities, speech and behavioral changes) is very well explained by epigenetic, environmental, and neo-peri-post-natal exposure factors. The AIM considers the permeability of the intestinal barrier and the BBB as components of the model. Because regression is observed in nearly 90% of people with ASD, it is necessary to ratify the whole body vision of ASD and the concept of an underlying systemic encephalopathy, where genes are a vulnerability risk and not a determining factor. An integrative approach is needed in ASD, and the three-hit model and dynamic and static encephalopathies should be considered.
*Responses to treatment(s) of CMP in individuals with ASD allow the identification of main groups and subgroups of people diagnosed with ASD. A paradigmatic shift in research is urgently needed, where the genetic vulnerabilities in these different subgroups are categorized by responses to CMP treatments. This information could give clues regarding the pathogenesis and etiology of non-syndromic and syndromic ASD, giving rise to the study of the mechanisms of generation of dynamic and static encephalopathy.
*A change is needed in the clinical approach for patients with an ASD diagnosis, where exhaustive clinical exploration and proper diagnosis and treatment of CMP are conducted considering the complexity of ASD (multi or hypermorbidity and heterogeneity). This is the first step to achieving health optimization, introducing the use of non-medical therapies and optimizing their results.
DECLARATIONS
Authors’ contributionsMade substantial contributions to conception and design of the search of the published literature and performed data analysis and interpretation: Ciolino A, Ferreira ML
Made substantial contribution to design of the study considering medical practice and interpretation of the published literature and its potential application in real world contexts, supervision of whole manuscript: Loyacono N
Availability of data and materialsNot applicable.
Financial support and sponsorshipNone.
Conflicts of interestFerreira ML is the mother of a young man of 22 years, diagnosed in 2003 with Autism. From Jun 2015 to Jun 2019 she was an ad-honorem Consultor in ASD to the Hospital de Clínicas José de San Martín (Buenos Aires Argentina), directed by Drs. Loyacono N and Iermoli R (Res. Directorio CONICET 3070/2015). A presentation for a potential agreement SANyTA (Director: Loyacono N)-CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas, from Argentina) is under development and will be presented for consideration to CONICET next months 2022/2023. Ciolino A and Loyacono N declared that they have no conflicts of interest.
Ethical approval and consent to participateNot applicable.
Consent for publicationNot applicable.
Copyright© The Author(s) 2022.
REFERENCES
1. Hoang N, Buchanan JA, Scherer SW. Heterogeneity in clinical sequencing tests marketed for autism spectrum disorders. NPJ Genom Med 2018;3:27.
2. Dias CM, Walsh CA. Recent advances in understanding the genetic architecture of autism. Annu Rev Genomics Hum Genet 2020;21:289-304.
3. Hertz-Picciotto I, Schmidt RJ, Krakowiak P. Understanding environmental contributions to autism: causal concepts and the state of science. Autism Res 2018;11:554-86.
4. Niesler B, Rappold GA. Emerging evidence for gene mutations driving both brain and gut dysfunction in autism spectrum disorder. Mol Psychiatry 2021;26:1442-4.
5. Ghrálaigh F, McCarthy E, Murphy DN, Gallagher L, Lopez LM. Brief report: evaluating the diagnostic yield of commercial gene panels in autism. J Autism Dev Disord 2022; doi: 10.1007/s10803-021-05417-7.
6. Zurita MF, Cárdenas PA, Sandoval ME, et al. Analysis of gut microbiome, nutrition and immune status in autism spectrum disorder: a case-control study in Ecuador. Gut Microbes 2020;11:453-64.
7. Myers SM, Challman TD, Bernier R, et al. Insufficient evidence for “autism-specific” genes. Am J Hum Genet 2020;106:587-95.
8. Lisik MZ, Gutmajster E, Sieroń AL. Anti-neuronal antibodies in patients with fragile X syndrome: is there a role of autoimmunity in its pathogenesis? Neurodegener Dis 2015;15:45-9.
9. Marco BD, Bonaccorso CM, Aloisi E, D'Antoni S, Catania MV. Neuro-inflammatory mechanisms in developmental disorders associated with intellectual disability and autism spectrum disorder: a neuro- immune perspective. CNS Neurol Disord Drug Targets 2016;15:448-63.
10. Barnhill K, Devlin M, Moreno HT, et al. Brief report: implementation of a specific carbohydrate diet for a child with autism spectrum disorder and fragile X syndrome. J Autism Dev Disord 2020;50:1800-8.
11. Nolan SO, Hodges SL, Binder MS, et al. Dietary rescue of adult behavioral deficits in the Fmr1 knockout mouse. PLoS One 2022;17:e0262916.
12. Napoli E, Schneider A, Wang JY, et al. Allopregnanolone treatment improves plasma metabolomic profile associated with gaba metabolism in fragile X-associated tremor/ataxia syndrome: a pilot study. Mol Neurobiol 2019;56:3702-13.
13. Song G, Napoli E, Wong S, et al. Altered redox mitochondrial biology in the neurodegenerative disorder fragile X-tremor/ataxia syndrome: use of antioxidants in precision medicine. Mol Med 2016;22:548-59.
14. Valenti D, de Bari L, De Filippis B, Henrion-Caude A, Vacca RA. Mitochondrial dysfunction as a central actor in intellectual disability-related diseases: an overview of Down syndrome, Autism, Fragile X and Rett syndrome. Neurosci Biobehav Rev 2014;46 Pt 2:202-17.
15. Müller M. Disturbed redox homeostasis and oxidative stress: potential players in the developmental regression in Rett syndrome. Neurosci Biobehav Rev 2019;98:154-63.
16. Carlsson T, Rosenqvist M, Butwicka A, et al. Association of cumulative early medical factors with autism and autistic symptoms in a population-based twin sample. Transl Psychiatry 2022;12:73.
18. Volk HE, Ames JL, Chen A, et al. Considering toxic chemicals in the etiology of autism. Pediatrics 2022;149:e2021053012.
19. Bougeard C, Picarel-Blanchot F, Schmid R, Campbell R, Buitelaar J. Prevalence of autism spectrum disorder and co-morbidities in children and adolescents: a systematic literature review. Front Psychiatry 2021;12:744709.
20. Leader G, Abberton C, Cunningham S, et al. Gastrointestinal symptoms in autism spectrum disorder: a systematic review. Nutrients 2022;14:1471.
21. Isaksson J, Ruchkin V, Aho N, Lundin Remnélius K, Marschik PB, Bölte S. Nonshared environmental factors in the aetiology of autism and other neurodevelopmental conditions: a monozygotic co-twin control study. Mol Autism 2022;13:8.
22. Wei H, Zhu Y, Wang T, Zhang X, Zhang K, Zhang Z. Genetic risk factors for autism-spectrum disorders: a systematic review based on systematic reviews and meta-analysis. J Neural Transm 2021;128:717-34.
23. David MM, Enard D, Ozturk A, et al. Comorbid analysis of genes associated with autism spectrum disorders reveals differential evolutionary constraints. PLoS One 2016;11:e0157937.
24. Diaz-Beltran L, Esteban FJ, Varma M, et al. Cross-disorder comparative analysis of comorbid conditions reveals novel autism candidate genes. BMC Genomics 2017;18:1-14.
25. Klein L, D'Urso S, Eapen V, Hwang LD, Lin PI. Exploring polygenic contributors to subgroups of comorbid conditions in autism spectrum disorder. Sci Rep 2022;12:3416.
26. Ferreira ML, Loyacono N. Rationale of an advanced integrative approach applied to autism spectrum disorder: review, discussion and proposal. J Pers Med 2021;11:514.
27. Cawthorpe D. A 16-year cohort analysis of autism spectrum disorder-associated morbidity in a pediatric population. Front Psychiatry 2018;9:635.
28. Ozonoff S, Gangi D, Hanzel EP, et al. Onset patterns in autism: variation across informants, methods, and timing. Autism Res 2018;11:788-97.
29. Rose S, Niyazov DM, Rossignol DA, Goldenthal M, Kahler SG, Frye RE. Clinical and molecular characteristics of mitochondrial dysfunction in autism spectrum disorder. Mol Diagn Ther 2018;22:571-93.
30. Goncalves MVM, Harger R, Braatz V, et al. Pediatric acute-onset neuropsychiatric syndrome (PANS) misdiagnosed as autism spectrum disorder. Immunol Lett 2018;203:52-3.
31. Shulyakova N, Andreazza AC, Mills LR, Eubanks JH. Mitochondrial dysfunction in the pathogenesis of rett syndrome: implications for mitochondria-targeted therapies. Front Cell Neurosci 2017;11:58.
32. Kreiman BL, Boles RG. State of the art of genetic testing for patients with autism: a practical guide for clinicians. Semin Pediatr Neurol 2020;34:100804.
33. Stafford CF, Sanchez-Lara PA. Impact of genetic and genomic testing on the clinical management of patients with autism spectrum disorder. Genes 2022;13:585.
34. Richards C, Jones C, Groves L, Moss J, Oliver C. Prevalence of autism spectrum disorder phenomenology in genetic disorders: a systematic review and meta-analysis. Lancet Psychiatry 2015;2:909-16.
35. Shimizu H, Morimoto Y, Yamamoto N, Tayama T, Ozawa H, Imamura A. Overlap between epilepsy and neurodevelopmental disorders: insights from clinical and genetic studies. In: Czuczwar SJ, editor. Epilepsy. Exon Publications; 2022. pp. 41-54.
36. Frye RE, Casanova MF, Fatemi SH, et al. Neuropathological mechanisms of seizures in autism spectrum disorder. Front Neurosci 2016;10:192.
37. Frye RE. Metabolic and mitochondrial disorders associated with epilepsy in children with autism spectrum disorder. Epilepsy Behav 2015;47:147-57.
38. Kuo SS, van der Merwe C, Fu JM, et al. Developmental variability in autism across 17 000 autistic individuals and 4000 siblings without an autism diagnosis: comparisons by cohort, intellectual disability, genetic etiology, and age at diagnosis. JAMA Pediatr 2022;176:915-23.
39. Mpoulimari I, Zintzaras E. Synthesis of genetic association studies on autism spectrum disorders using a genetic model-free approach. Psychiatr Genet 2022;32:91-104.
40. Doi M, Li M, Usui N, Shimada S. Genomic strategies for understanding the pathophysiology of autism spectrum disorder. Front Mol Neurosci 2022;15:930941.
41. Casanova MF, Frye RE, Gillberg C, Casanova EL. Editorial: comorbidity and autism spectrum disorder. Front Psychiatry 2020;11:617395.
42. Autistica. Personal tragedies, public crisis. Available from: https://www.autistica.org.uk/downloads/files/Personal-tragedies-public-crisis-ONLINE.pdf [Last accessed on 27 Dec 2022].
43. Wills J, Evans Y. Health and service provision for people with autism spectrum disorders: a survey of parents in the United Kingdom. Available from: https://www.thinkingautism.org.uk/wp-content/uploads/Health__Service_Provision_for_People_with_ASD_March2016.pdf [Last accessed on 27 Dec 2022].
44. Lord C, Charman T, Havdahl A. The Lancet Commission on the future of care and clinical research in autism. Lancet Comm 2021;399:271-334.
45. Qiu S, Qiu Y, Li Y, Cong X. Genetics of autism spectrum disorder: an umbrella review of systematic reviews and meta-analyses. Transl Psychiatry 2022;12:249.
46. Panisi C, Guerini FR, Abruzzo PM, et al. Autism spectrum disorder from the womb to adulthood: suggestions for a paradigm shift. J Pers Med 2021 ;11:70.
47. MDPI. Special issue “a personalized medicine approach to the diagnosis and management of autism spectrum disorder”. Available from: https://www.mdpi.com/journal/jpm/special_issues/Personalized_Medicine_Approach_ASD [Last accessed on 27 Dec 2022].
48. MDPI. Special issue “a personalized medicine approach to the diagnosis and management of autism spectrum disorder: 2022”. Available from: https://www.mdpi.com/journal/jpm/special_issues/asd_personalized [Last accessed on 27 Dec 2022].
49. Whiteley P, Marlow B, Kapoor RR, Blagojevic-Stokic N, Sala R. Autoimmune encephalitis and autism spectrum disorder. Front Psychiatry 2021;12:775017.
50. Whiteley P, Carr K, Shattock P. Research, clinical, and sociological aspects of autism. Front Psychiatry 2021;12:481546.
51. Chen YJ, Duku E, Georgiades S. Rethinking autism intervention science: a dynamic perspective. Front Psychiatry 2022;13:827406.
52. Erbescu A, Papuc SM, Budisteanu M, Arghir A, Neagu M. Re-emerging concepts of immune dysregulation in autism spectrum disorders. Front Psychiatry 2022;13:1006612.
53. Jensen AR, Lane AL, Werner BA, McLees SE, Fletcher TS, Frye RE. Modern biomarkers for autism spectrum disorder: future directions. Mol Diagn Ther 2022;26:483-95.
55. Paulsen B, Velasco S, Kedaigle AJ, et al. Autism genes converge on asynchronous development of shared neuron classes. Nature 2022;602:268-73.
56. Shen L, Zhang H, Lin J, et al. A combined proteomics and metabolomics profiling to investigate the genetic heterogeneity of autistic children. Mol Neurobiol 2022;59:3529-45.
57. Roe K. Autism spectrum disorder initiation by inflammation-facilitated neurotoxin transport. Neurochem Res 2022;47:1150-65.
58. Ohashi K, Fukuhara S, Miyachi T, et al. Comprehensive genetic analysis of non-syndromic autism spectrum disorder in clinical settings. J Autism Dev Disord 2021;51:4655-62.
59. Yu KH, Palmer N, Fox K, et al. The phenotypical implications of immune dysregulation in fragile X syndrome. Eur J Neurol 2020;27:590-3.
Cite This Article
Export citation file: BibTeX | RIS
OAE Style
Ciolino A, Ferreira ML, Loyacono N. The importance of genetics in an advanced integrative model of autism spectrum disorder. J Transl Genet Genom 2022;6:429-42. http://dx.doi.org/10.20517/jtgg.2022.15
AMA Style
Ciolino A, Ferreira ML, Loyacono N. The importance of genetics in an advanced integrative model of autism spectrum disorder. Journal of Translational Genetics and Genomics. 2022; 6(4): 429-42. http://dx.doi.org/10.20517/jtgg.2022.15
Chicago/Turabian Style
Ciolino, Andrés, María Luján Ferreira, Nicolás Loyacono. 2022. "The importance of genetics in an advanced integrative model of autism spectrum disorder" Journal of Translational Genetics and Genomics. 6, no.4: 429-42. http://dx.doi.org/10.20517/jtgg.2022.15
ACS Style
Ciolino, A.; Ferreira ML.; Loyacono N. The importance of genetics in an advanced integrative model of autism spectrum disorder. J. Transl. Genet. Genom. 2022, 6, 429-42. http://dx.doi.org/10.20517/jtgg.2022.15
About This Article
Special Issue
Copyright
Data & Comments
Data

 Cite This Article 19 clicks
Cite This Article 19 clicks










Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.